Page 747 - Corrosion Engineering Principles and Practice
P. 747
698 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 699
0.5
0.5
0.4
0.4
0.3
Penetration (mm) 0.3
2
0.2 100 ppm H S
10 ppm H S
2
1 ppm H S
2
0.2
0.1
0.1
0.0
260 310 360 410 460 510
Temperature (°C)
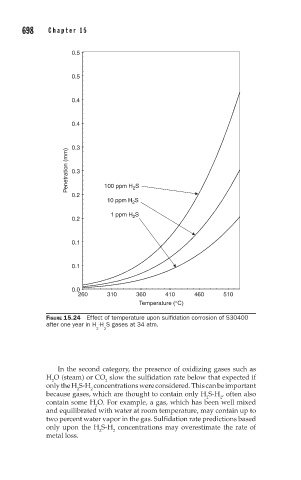
FIGURE 15.24 Effect of temperature upon sulfidation corrosion of S30400
after one year in H -H S gases at 34 atm.
2 2
In the second category, the presence of oxidizing gases such as
H O (steam) or CO slow the sulfidation rate below that expected if
2
2
only the H S-H concentrations were considered. This can be important
2
2
because gases, which are thought to contain only H S-H , often also
2
2
contain some H O. For example, a gas, which has been well mixed
2
and equilibrated with water at room temperature, may contain up to
two percent water vapor in the gas. Sulfidation rate predictions based
only upon the H S-H concentrations may overestimate the rate of
2
2
metal loss.