Page 745 - Corrosion Engineering Principles and Practice
P. 745
696 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 697
5.0
4.5
4.0
3.5
3.0
Penetration (mm) 2.5
100 ppm H S
2
2.0
S
10 ppm H 2
S
1 ppm H 2
1.5
1.0
0.5
0.0
260 310 360 410 460 510
Temperature (°C)
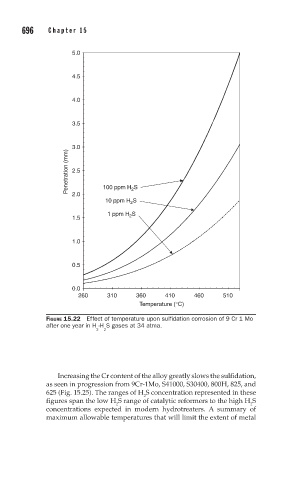
FIGURE 15.22 Effect of temperature upon sulfidation corrosion of 9 Cr 1 Mo
after one year in H -H S gases at 34 atma.
2 2
Increasing the Cr content of the alloy greatly slows the sulfidation,
as seen in progression from 9Cr-1Mo, S41000, S30400, 800H, 825, and
625 (Fig. 15.25). The ranges of H S concentration represented in these
2
figures span the low H S range of catalytic reformers to the high H S
2
2
concentrations expected in modern hydrotreaters. A summary of
maximum allowable temperatures that will limit the extent of metal