Page 749 - Corrosion Engineering Principles and Practice
P. 749
700 C h a p t e r 1 5 H i g h - Te m p e r a t u r e C o r r o s i o n 701
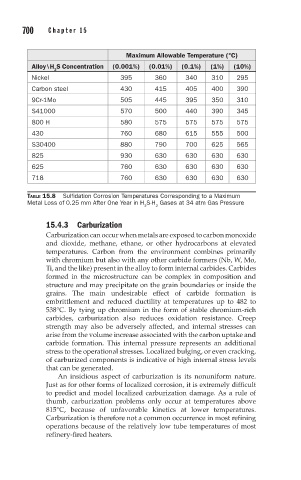
Maximum Allowable Temperature (°C)
Alloy\H S Concentration (0.001%) (0.01%) (0.1%) (1%) (10%)
2
Nickel 395 360 340 310 295
Carbon steel 430 415 405 400 390
9Cr-1Mo 505 445 395 350 310
S41000 570 500 440 390 345
800 H 580 575 575 575 575
430 760 680 615 555 500
S30400 880 790 700 625 565
825 930 630 630 630 630
625 760 630 630 630 630
718 760 630 630 630 630
TABLE 15.8 Sulfidation Corrosion Temperatures Corresponding to a Maximum
Metal Loss of 0.25 mm After One Year in H S-H Gases at 34 atm Gas Pressure
2 2
15.4.3 Carburization
Carburization can occur when metals are exposed to carbon monoxide
and dioxide, methane, ethane, or other hydrocarbons at elevated
temperatures. Carbon from the environment combines primarily
with chromium but also with any other carbide formers (Nb, W, Mo,
Ti, and the like) present in the alloy to form internal carbides. Carbides
formed in the microstructure can be complex in composition and
structure and may precipitate on the grain boundaries or inside the
grains. The main undesirable effect of carbide formation is
embrittlement and reduced ductility at temperatures up to 482 to
538°C. By tying up chromium in the form of stable chromium-rich
carbides, carburization also reduces oxidation resistance. Creep
strength may also be adversely affected, and internal stresses can
arise from the volume increase associated with the carbon uptake and
carbide formation. This internal pressure represents an additional
stress to the operational stresses. Localized bulging, or even cracking,
of carburized components is indicative of high internal stress levels
that can be generated.
An insidious aspect of carburization is its nonuniform nature.
Just as for other forms of localized corrosion, it is extremely difficult
to predict and model localized carburization damage. As a rule of
thumb, carburization problems only occur at temperatures above
815°C, because of unfavorable kinetics at lower temperatures.
Carburization is therefore not a common occurrence in most refining
operations because of the relatively low tube temperatures of most
refinery-fired heaters.