Page 86 - Coulson Richardson's Chemical Engineering Vol.6 Chemical Engineering Design 4th Edition
P. 86
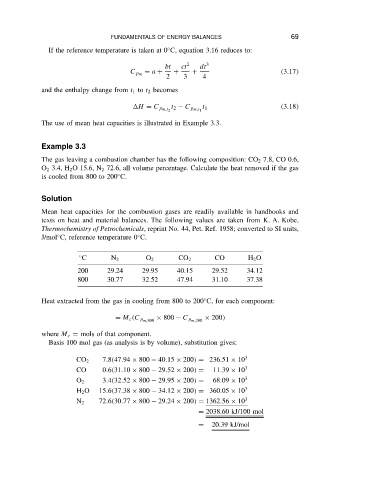
FUNDAMENTALS OF ENERGY BALANCES
Ž
If the reference temperature is taken at 0 C, equation 3.16 reduces to:
bt ct 2 dt 3 69
D a C C C 3.17
C p m
2 3 4
and the enthalpy change from t 1 to t 2 becomes
t 1 3.18
H D C p m,t 2 t 2 C p m,t 1
The use of mean heat capacities is illustrated in Example 3.3.
Example 3.3
The gas leaving a combustion chamber has the following composition: CO 2 7.8, CO 0.6,
O 2 3.4, H 2 O 15.6, N 2 72.6, all volume percentage. Calculate the heat removed if the gas
Ž
is cooled from 800 to 200 C.
Solution
Mean heat capacities for the combustion gases are readily available in handbooks and
texts on heat and material balances. The following values are taken from K. A. Kobe,
Thermochemistry of Petrochemicals, reprint No. 44, Pet. Ref. 1958; converted to SI units,
Ž
Ž
J/mol C, reference temperature 0 C.
Ž
C N 2 O 2 CO 2 CO H 2 O
200 29.24 29.95 40.15 29.52 34.12
800 30.77 32.52 47.94 31.10 37.38
Ž
Heat extracted from the gas in cooling from 800 to 200 C, for each component:
ð 200
D M c C p m,800 ð 800 C p m,200
where M c D mols of that component.
Basis 100 mol gas (as analysis is by volume), substitution gives:
7.8 47.94 ð 800 40.15 ð 200 D 236.51 ð 10 3
CO 2
CO 0.6 31.10 ð 800 29.52 ð 200 D 11.39 ð 10 3
O 2 3.4 32.52 ð 800 29.95 ð 200 D 68.09 ð 10 3
H 2 O 15.6 37.38 ð 800 34.12 ð 200 D 360.05 ð 10 3
N 2 72.6 30.77 ð 800 29.24 ð 200 D 1362.56 ð 10 3
D 2038.60 kJ/100 mol
D 20.39 kJ/mol