Page 87 - Coulson Richardson's Chemical Engineering Vol.6 Chemical Engineering Design 4th Edition
P. 87
70
CHEMICAL ENGINEERING
3.7. THE EFFECT OF PRESSURE ON HEAT CAPACITY
The data on heat capacities given in the handbooks, and in Appendix A, are, usually for
the ideal gas state. Equation 3.13a should be written as:
Ž
2
C D a C bT C cT C dT 3 3.19
p
where the superscript Ž refers to the ideal gas state.
The ideal gas values can be used for the real gases at low pressures. At high pressures
the effect of pressure on the specific heat may be appreciable.
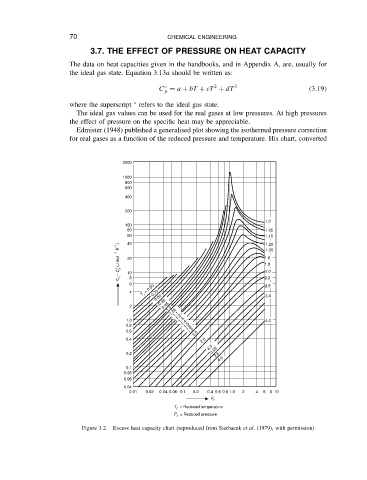
Edmister (1948) published a generalised plot showing the isothermal pressure correction
for real gases as a function of the reduced pressure and temperature. His chart, converted
2000
1000
800
600
400
200
1.0
100
80 1.05
60 1.10
−1 ) 40 1.20
−1 K 20 1.30
C p − C p (J mol 1.8
1.6
° 10 2.0
6 8 2.2
T r = 0.60
2.5
4 0.70
0.80 3.0
2 0.90 0.95 1.0 1.05
1.0 1.1 1.2 1.3 1.4 4.0
0.8 1.5 1.6
0.6 1.8
2.0
0.4 2.5
3.0
0.2 3.25 3.5
4.0
0.1
0.08
0.06
0.04
0.01 0.02 0.04 0.06 0.1 0.2 0.4 0.6 0.8 1.0 2 4 6 8 10
P r
T r = Reduced temperature
P r = Reduced pressure
Figure 3.2. Excess heat capacity chart (reproduced from Sterbacek et al. (1979), with permission)