Page 92 - Coulson Richardson's Chemical Engineering Vol.6 Chemical Engineering Design 4th Edition
P. 92
75
FUNDAMENTALS OF ENERGY BALANCES
Himmelbau (1995) or any of the general texts on material and energy balances listed at the
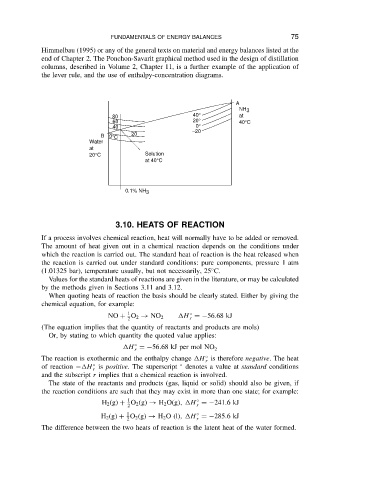
end of Chapter 2. The Ponchon-Savarit graphical method used in the design of distillation
columns, described in Volume 2, Chapter 11, is a further example of the application of
the lever rule, and the use of enthalpy-concentration diagrams.
A
NH 3
40° at
80
60 20° 40°C
40 0°
20 −20
B 0°C
Water
at
20°C Solution
at 40°C
0.1% NH 3
3.10. HEATS OF REACTION
If a process involves chemical reaction, heat will normally have to be added or removed.
The amount of heat given out in a chemical reaction depends on the conditions under
which the reaction is carried out. The standard heat of reaction is the heat released when
the reaction is carried out under standard conditions: pure components, pressure 1 atm
Ž
(1.01325 bar), temperature usually, but not necessarily, 25 C.
Values for the standard heats of reactions are given in the literature, or may be calculated
by the methods given in Sections 3.11 and 3.12.
When quoting heats of reaction the basis should be clearly stated. Either by giving the
chemical equation, for example:
1 Ž
NO C O 2 ! NO 2 H D 56.68 kJ
2 r
(The equation implies that the quantity of reactants and products are mols)
Or, by stating to which quantity the quoted value applies:
Ž
H D 56.68 kJ per mol NO
r 2
Ž
The reaction is exothermic and the enthalpy change H is therefore negative. The heat
r
Ž
of reaction H is positive. The superscript Ž denotes a value at standard conditions
r
and the subscript r implies that a chemical reaction is involved.
The state of the reactants and products (gas, liquid or solid) should also be given, if
the reaction conditions are such that they may exist in more than one state; for example:
Ž
1
H 2 (g) C O 2 (g) ! H 2 O(g),H D 241.6kJ
2 r
Ž
1
H 2 (g) C O 2 (g) ! H 2 O(l),H D 285.6kJ
2 r
The difference between the two heats of reaction is the latent heat of the water formed.