Page 122 - Distillation theory
P. 122
P1: FCH/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521832772c04 CB644-Petlyuk-v1 June 11, 2004 17:49
96 Trajectories of Thermodynamically Reversible Distillation
2 2
α α
23
23
a) b)
x B
x B
α 13 α 13
1 α 23 4 1 α 23 4
α 2 α α
23 α 13 23 13
13 α 13
23
3 c) 3
α
13 α
1 23 4
α 23
α
13 23
3
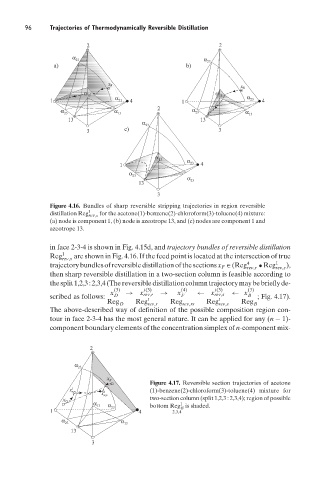
Figure 4.16. Bundles of sharp reversible stripping trajectories in region reversible
distillation Reg 1 rev,s for the acetone(1)-benzene(2)-chloroform(3)-toluene(4) mixture:
(a) node is component 1, (b) node is azeotrope 13, and (c) nodes are component 1 and
azeotrope 13.
in face 2-3-4 is shown in Fig. 4.15d, and trajectory bundles of reversible distillation
Reg 1 are shown in Fig. 4.16. If the feed point is located at the intersection of true
rev,s
trajectorybundlesofreversibledistillationofthesections x F ∈ (Reg 4 rev,r • Reg 1 rev,s ),
then sharp reversible distillation in a two-section column is feasible according to
thesplit1,2,3:2,3,4(Thereversibledistillationcolumntrajectorymaybebrieflyde-
(3) t(3) (4) t(3) (3)
x → x rev,r → x ← x rev,s ← x
scribed as follows: D t F t B ; Fig. 4.17).
Reg Reg Reg Reg Reg
D rev,r rev,rs rev,s B
The above-described way of definition of the possible composition region con-
tour in face 2-3-4 has the most general nature. It can be applied for any (n − 1)-
component boundary elements of the concentration simplex of n-component mix-
2
α 23
x B
Figure 4.17. Reversible section trajectories of acetone
x t (1)-benzene(2)-chloroform(3)-toluene(4) mixture for
rev t
x rev
two-section column (split 1,2,3 : 2,3,4); region of possible
α 1
x D
13 α 23 bottom Reg is shaded.
B
1 4 2,3,4
α α
23 13
13
3