Page 123 - Distillation theory
P. 123
P1: FCH/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521832772c04 CB644-Petlyuk-v1 June 11, 2004 17:49
4.6 Diagrams of Extractive Reversible Distillation for Three-Component Mixtures 97
tures. It is necessary to determine phase equilibrium coefficients of all the compo-
nents in points of all the edges of the (n − 1)-component boundary element under
consideration. After that, all possible product points x D and x B at each edge are
defined according to Eq. (4.19) or (4.20) for all tear-off points x t from this edge
rev
into three-component boundary element, containing the component absent in the
(n − 1)-component boundary element under consideration.
4.6. Diagrams of Extractive Reversible Distillation for
Three-Component Mixtures
4.6.1. Condition in Tear-Off Points of the Extractive Reversible
Distillation Trajectories
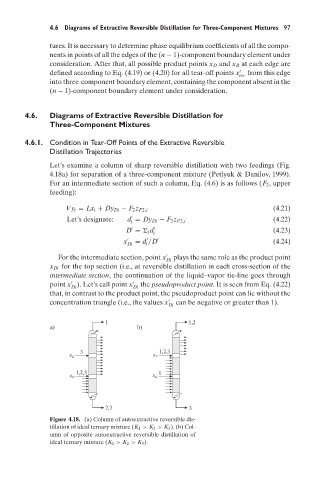
Let’s examine a column of sharp reversible distillation with two feedings (Fig.
4.18a) for separation of a three-component mixture (Petlyuk & Danilov, 1999).
For an intermediate section of such a column, Eq. (4.6) is as follows (F 2 , upper
feeding):
Vy i = Lx i + Dy Di − F 2 z F2,i (4.21)
Let’s designate: d = Dy Di − F 2 z F2,i (4.22)
i
D =
i d i (4.23)
x Di = d /D (4.24)
i
For the intermediate section, point x Di plays the same role as the product point
x Di for the top section (i.e., at reversible distillation in each cross-section of the
intermediate section, the continuation of the liquid–vapor tie-line goes through
point x ). Let’s call point x the pseudoproduct point. It is seen from Eq. (4.22)
Di Di
that, in contrast to the product point, the pseudoproduct point can lie without the
concentration triangle (i.e., the values x can be negative or greater than 1).
Di
1 1,2
a) b)
3 1, ,23
x E x F
1, ,23 1
x F x E
2,3 3
Figure 4.18. (a) Column of autoextractive reversible dis-
tillation of ideal ternary mixture (K 1 > K 2 > K 3 ). (b) Col-
umn of opposite autoextractive reversible distillation of
ideal ternary mixture (K 1 > K 2 > K 3 ).