Page 334 - Distillation theory
P. 334
P1: JPJ/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521820928c08 CB644-Petlyuk-v1 June 11, 2004 20:20
308 Synthesis of Separation Flowsheets
x
E
) 1 (
x 2
D
2
) 2 ( ) 3 (
x x
a) b) D D
x ) 0 ( x ) 1 (
F F
321
23 ) 3 (
α 23 x
D
x D ) 2 ( = x F ) 3 ( ) 3 (
231 x ) 1 ( x ) 2 ( x F
) 1 ( ) 0 ( F F
x F + E x F
x B ) 1 ( = x F ) 2 ( α 13
213 x ) 3 ( x ) 1 (
B
B 1
1 3
13 3
) 2 (
x = x
B E
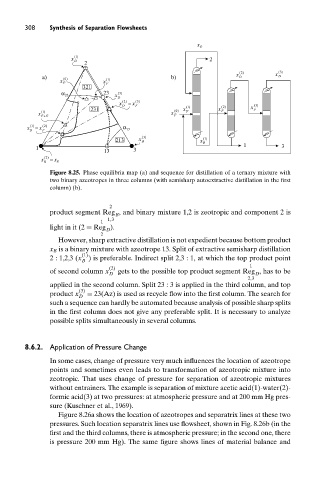
Figure 8.25. Phase equilibria map (a) and sequence for distillation of a ternary mixture with
two binary azeotropes in three columns (with semisharp autoextractive distillation in the first
column) (b).
2
product segment Reg , and binary mixture 1,2 is zeotropic and component 2 is
B
1,3
1
light in it (2 ≡ Reg ).
D
2
However, sharp extractive distillation is not expedient because bottom product
x B is a binary mixture with azeotrope 13. Split of extractive semisharp distillation
(1)
2 : 1,2,3 (x ) is preferable. Indirect split 2,3 : 1, at which the top product point
B
1
(2)
of second column x gets to the possible top product segment Reg , has to be
D D
2,3
applied in the second column. Split 23 : 3 is applied in the third column, and top
(3)
product x = 23(Az) is used as recycle flow into the first column. The search for
D
such a sequence can hardly be automated because analysis of possible sharp splits
in the first column does not give any preferable split. It is necessary to analyze
possible splits simultaneously in several columns.
8.6.2. Application of Pressure Change
In some cases, change of pressure very much influences the location of azeotrope
points and sometimes even leads to transformation of azeotropic mixture into
zeotropic. That uses change of pressure for separation of azeotropic mixtures
without entrainers. The example is separation of mixture acetic acid(1)-water(2)-
formic acid(3) at two pressures: at atmospheric pressure and at 200 mm Hg pres-
sure (Kuschner et al., 1969).
Figure 8.26a shows the location of azeotropes and separatrix lines at these two
pressures. Such location separatrix lines use flowsheet, shown in Fig. 8.26b (in the
first and the third columns, there is atmospheric pressure; in the second one, there
is pressure 200 mm Hg). The same figure shows lines of material balance and