Page 336 - Distillation theory
P. 336
P1: JPJ/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521820928c08 CB644-Petlyuk-v1 June 11, 2004 20:20
310 Synthesis of Separation Flowsheets
2 ) 2 ( ) 1 (
x x
D D
12 2
a)
x D ) 1 ( = x F ) 2 ( x ) 0 ( x F ) 1 ( x F ) 2 (
x F ) 0 ( F
) 1 (
x x ) 2 ( 1 x ) 2 (
B B B
1 3
x ) 1 (
F
2 ) 1 (
x D
12 2 x D ) 2 (
b)
x ) 1 ( x ) 2 (
x ) 0 ( F F
x ) 0 ( F
F
) 2 ( x ) 1 (
x x ) 2 ( B
B D 1
1 ) 1 ( 3
x ) 1 ( = x ) 2 ( x
B F F
2
2
x D ) 2 (
12 x D ) 1 (
x D ) 1 ( = x F 1 ) 2 ( ) 2 (
c) x F 1
x F ) 1 ( x ) 2 (
x ) 0 ( x ) 0 ( M
F F
x F 2 ) 2 (
x ) 2 ( x ) 2 ( x ) 1 (
B M B
1 ) 1 ( 3
x B ) 1 ( = x F 2 ) 2 ( x F 1
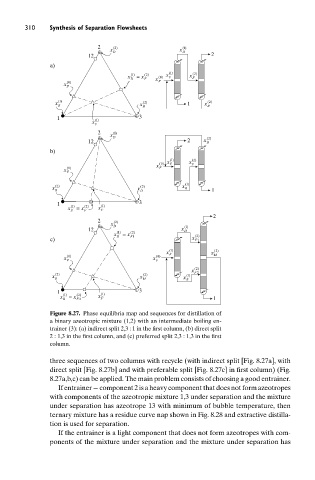
Figure 8.27. Phase equilibria map and sequences for distillation of
a binary azeotropic mixture (1,2) with an intermediate boiling en-
trainer (3): (a) indirect split 2,3 : 1 in the first column, (b) direct split
2 : 1,3 in the first column, and (c) preferred split 2,3 : 1,3 in the first
column.
three sequences of two columns with recycle (with indirect split [Fig. 8.27a], with
direct split [Fig. 8.27b] and with preferable split [Fig. 8.27c] in first column) (Fig.
8.27a,b,c) can be applied. The main problem consists of choosing a good entrainer.
If entrainer − component 2 is a heavy component that does not form azeotropes
with components of the azeotropic mixture 1,3 under separation and the mixture
under separation has azeotrope 13 with minimum of bubble temperature, then
ternary mixture has a residue curve nap shown in Fig. 8.28 and extractive distilla-
tion is used for separation.
If the entrainer is a light component that does not form azeotropes with com-
ponents of the mixture under separation and the mixture under separation has