Page 59 - Distillation theory
P. 59
P1: FCH/FFX P2: FCH/FFX QC: VINOD/IYP T1: FCH
0521820928c02 CB644-Petlyuk-v1 June 11, 2004 17:58
2.7 Adiabatic, Nonadiabatic, and Reversible Distillation 33
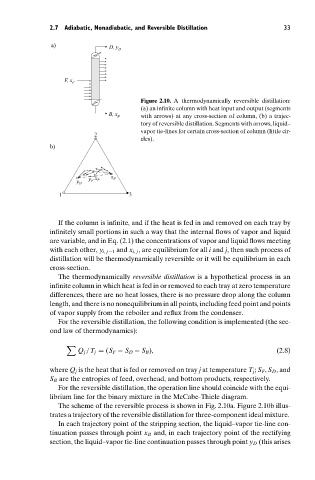
a) D, y
D
F, x
F
Figure 2.10. A thermodynamically reversible distillation:
(a) an infinite column with heat input and output (segments
B, x B with arrows) at any cross-section of column, (b) a trajec-
tory of reversible distillation. Segments with arrows, liquid–
vapor tie-lines for certain cross-section of column (little cir-
2
cles).
b)
x B
y F x F
y D
1 3
If the column is infinite, and if the heat is fed in and removed on each tray by
infinitely small portions in such a way that the internal flows of vapor and liquid
are variable, and in Eq. (2.1) the concentrations of vapor and liquid flows meeting
with each other, y i, j−1 and x i, j , are equilibrium for all i and j, then such process of
distillation will be thermodynamically reversible or it will be equilibrium in each
cross-section.
The thermodynamically reversible distillation is a hypothetical process in an
infinite column in which heat is fed in or removed to each tray at zero temperature
differences, there are no heat losses, there is no pressure drop along the column
length, and there is no nonequilibrium in all points, including feed point and points
of vapor supply from the reboiler and reflux from the condenser.
For the reversible distillation, the following condition is implemented (the sec-
ond law of thermodynamics):
Q j /T j = (S F − S D − S B ), (2.8)
where Q j is the heat that is fed or removed on tray j at temperature T j ; S F , S D , and
S B are the entropies of feed, overhead, and bottom products, respectively.
For the reversible distillation, the operation line should coincide with the equi-
librium line for the binary mixture in the McCabe-Thiele diagram.
The scheme of the reversible process is shown in Fig. 2.10a. Figure 2.10b illus-
trates a trajectory of the reversible distillation for three-component ideal mixture.
In each trajectory point of the stripping section, the liquid–vapor tie-line con-
tinuation passes through point x B and, in each trajectory point of the rectifying
section, the liquid–vapor tie-line continuation passes through point y D (this arises