Page 306 - Dust Explosions in the Process Industries
P. 306
Propagation of Flames in Dust Clouds 275
The question of what are the true laminar burning velocities for coal dust clouds to
some extent remains unanswered. The true peak values are probably somewhat higher
than 0.35 m/s but certainly lower than the exceptional value of 0.86 m/s measured by
Ghosh, Basu, and Roy (1957) (see Table 4.5, observation 2).
In a comprehensive investigation comprising several types of dusts, Ballal (1983)
determined the laminar burning velocity in clouds of coal dust in air under zero gravity
conditions, using a free-fallexplosion tube. For a coal dust of 8 pm surface-volumediam-
eter (032) and 13.8% volatile matter, the maximumburning velocity of 0.11 m/s was found
for dust concentrations close to the stoichiometric,that is, 210 g/m3.For coals of higher
volatile contents, the maximum values were about 0.25 m/s (40% volatiles and D3, =
12pm),0.17 m/s (27% volatiles and 032 = 11pm), and 0.12 m/s (37% volatiles and 032=
47 pm). The experimentalconcentration range did not extend beyond the stoichiometric
concentration for which the maximum values were obtained. However, the trend of the
experimental burning velocity-versus-dust concentration curves indicated that even
higheir burning velocities would have been found for dust concentrations somewhat
higher than the stoichiometric.It is interesting to note that the burning velocities meas-
ured by Ballal for codair under zero gravity conditions are close to those found under
normal gravity conditions by Smoot and Horton (1977) and Horton et al. (1977).
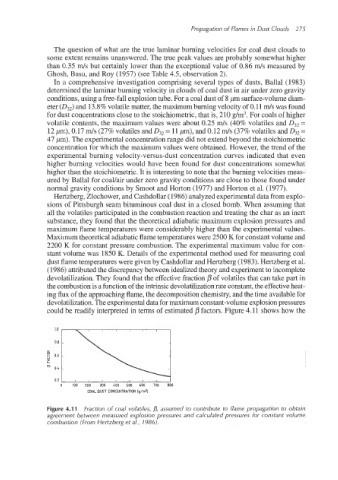
Hertzberg,Zlochower, and Cashdollar(1986) analyzed experimentaldata from explo-
sions of Pittsburgh seam bituminous coal dust in a closed bomb. When assuming that
all the volatiles participated in the combustion reaction and treating the char as an inert
substance, they found that the theoretical adiabatic maximum explosion pressures and
maximum flame temperatures were considerably higher than the experimental values.
Maximum theoretical adiabatic flame temperatures were 2500 K for constant volume and
2200 K for constant pressure combustion. The experimental maximum value for con-
stant volume was 1850 K. Details of the experimentalmethod used for measuring coal
dust flame temperatures were given by Cashdollarand Hertzberg (1983). Hertzberg et al.
(1986) attributed the discrepancy between idealized theory and experiment to incomplete
devolatilization.They found that the effective fraction p of volatiles that can take part in
the combustion is a function of the intrinsic devolatilizationrate constant,the effectiveheat-
ing flux of the approaching flame, the decompositionchemistry, and the time availablefor
devolatilization.The experimentaldata for maximum constant-volumeexplosionpressures
couBd be readily interpreted in terms of estimated j3 factors. Figure 4.11 shows how the
0.2 I
0 100 200 300 400 500 600 700 IO
COAL OUST CONCENTRATION [g/m31
Figure 4.1 1 Fraction of coal volatiles, D, assumed to contribute to flame propagation to obtain
agreement between measured explosion pressures and calculated pressures for constant volume
combustion (From Hertzberg et a/., 1986).