Page 302 - Dust Explosions in the Process Industries
P. 302
Propagation of Flames in Dust Clouds 271
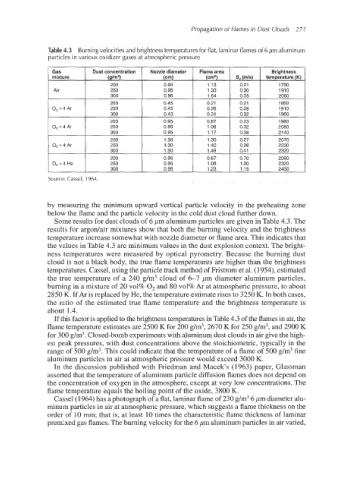
Table 4.3 Burningvelocities and brightness temperatures for flat, laminar flames of 6 pm aluminum
particles in various oxidizer gases at atmospheric pressure
Source: Cassel, 1964.
by measuring the minimum upward vertical particle velocity in the preheating zone
below the flame and the particle velocity in the cold dust cloud further down.
Some results for dust clouds of 6 pm aluminum particles are given in Table 4.3. The
results for argonlair mixtures show that both the burning velocity and the brightness
temperature increase somewhat with nozzle diameter or flame area. This indicates that
the values in Table 4.3 are minimum values in the dust explosion context. The bright-
ness temperatures were measured by optical pyrometry. Because the burning dust
cloud is not a black body, the true flame temperatures are higher than the brightness
temperatures. Cassel,using the particle track method of Fristrom et al. (1954),estimated
the true temperature of a 240 g/m3 cloud of 6-7 pm diameter aluminum particles,
burning in a mixture of 20 vol% O2and 80 vol% Ar at atmospheric pressure, to about
2850 K. If AI is replaced by He, the temperature estimate rises to 3250 K. In both cases,
the ratio of the estimated true flame temperature and the brightness temperature is
about 1.4.
If this factor is appliedto the brightness temperatures in Table 4.3 of the flames in air,the
flame temperature estimates are 2500 K for 200 g/m3,2670 K for 250 g/m3,and 2900 K
for 300 g/m3.Closed-bombexperimentswith aluminum dust clouds in air give the high-
est peak pressures, with dust concentrations above the stoichiometric, typically in the
range of 500 g/m3.This could indicate that the temperature of a flame of 500 g/m3fine
aluminum particles in air at atmosphericpressure would exceed 3000 K.
In the discussion published with Friedman and Macek’s (1963) paper, Glassman
asserted that the temperature of aluminumparticle diffusion flames does not depend on
the concentration of oxygen in the atmosphere, except at very low concentrations.The
flame temperature equals the boiling point of the oxide, 3800 K.
Cassel(l964) has a photograph of a flat, laminar flame of 230 g/m36 pm diameter alu-
minum particles in air at atmosphericpressure, which suggests a flame thickness on the
order of 10 mm; that is, at least 10 times the characteristic flame thickness of laminar
premixed gas flames.The burning velocity for the 6 pm aluminum particles in air varied,