Page 303 - Dust Explosions in the Process Industries
P. 303
272 Dust Explosions in the Process industries
as seen from Table 4.3, with the dust concentration,being 0.21 m/s for 200 g/m3and 0.35
m/s for 300 g/m3.
Other experimentsby Cassel(l964) showed that the burning velocity of aluminudair
clouds also increased with decreasing particle size. At 200 g/m3,it was roughly 0.2 m/s
for a “<30pm” atomized aluminumpowder and 0.4 m/s for ‘‘40 pm” quality.The latter
value agrees favorablywith the maximum value of 0.42 m/s determined by Ballal(l983)
for aluminum of a volume surface mean diameter (032)of 10 pm. The maximum flame
speed occurred close to the stoichiometric concentration 310 g/m3.Ballal (1983) con-
ducted his sophisticatedexperimentsin a special vertical explosion tube during free fall
(zero gravity conditions), and it is interesting to observe that, for particle sizes of about
10pm, gravitational effects did not seem to play a dominatingrole in the laminar flame
propagation through aluminum dust clouds.
Gardiner, Caird, and Bardon (1988) studied flame propagationin comparatively small,
electrostatically suspended clouds of 20 pm volume surface mean diameter aluminum
particles in air in a small semi-closed cylindrical vessel and found maximum flame
speeds in excess of 2.0 m/s.
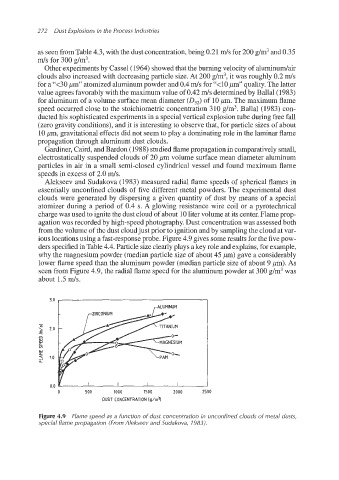
Alekseev and Sudakova (1983) measured radial flame speeds of spherical flames in
essentially unconfined clouds of five different metal powders. The experimental dust
clouds were generated by dispersing a given quantity of dust by means of a special
atomizer during a period of 0.4 s. A glowing resistance wire coil or a pyrotechnical
charge was used to ignite the dust cloud of about 10liter volume at its center. Flameprop-
agation was recorded by high-speedphotography. Dust concentrationwas assessed both
from the volume of the dust cloudjust prior to ignition and by samplingthe cloud at var-
ious locationsusing a fast-response probe. Figure 4.9 gives some results for the five pow-
ders specified in Table 4.4. Particle size clearly plays a key role and explains, for example,
why the magnesium powder (median particle size of about 45 pm) gave a considerably
lower flame speed than the aluminum powder (median particle size of about 9 pm). As
seen from Figure 4.9, the radial flame speed for the aluminum powder at 300 g/m3was
about 1.5 m/s.
3.0
,-ALUMINUM
0.0 y I I I I
0 500 1000 1500 2000 2500
DUST CONCENTRATION [g/m?
Figure 4.9 Flame speed as a function of dust concentration in unconfined clouds of metal dusts,
special flame propagation (From Alekseev and Sudakova, 1983).