Page 338 - Dust Explosions in the Process Industries
P. 338
Propagation of Flames in Dust Clouds 307
minimum explosible concentration for a given dust inevitably implies the use of some
arbitrary criterion of explosion, as a finite minimum pressure rise at constant volume or
a minimum finite extent of flame propagation at constant pressure. A transition range rep-
resenting a factor of 2 of average dust concentrations, from the first sign of self-sustained
flame to extensive flame propagation, is probably typical of many experiments.
Another aspectthat needs considerationis the influence of the settling of particles due
to gravity on the minimum explosible dust concentration.Burgoyne (1963), discussing
the minimum explosible concentrationof clouds of liquid droplets, distinguishedbetween
“static” and “kinetic” minimum explosibleconcentrationsC, and Ck If the drops are suf-
ficiently large for their gravitational sedimentationvelocities v,to be significant and S,
is the upward burning velocity in the drop cloud, then C, and C, differ according to
(4.65)
This equation should also be applicable to solid particles that volatilize or pyrolyze in
the preheating zone of the flame front, that is, organic materials and coals.
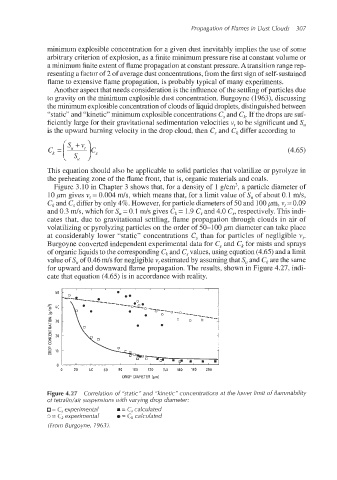
Figure 3.10 in Chapter 3 shows that, for a density of 1 g/cm‘, a particle diameter of
10 pm gives v,= 0.004 ds, which means that, for a limit value of S, of about 0.1 ds,
C, and C, differ by only 4%.However, for particle diametersof 50 and 100pm, v,= 0.09
and 0.3 ds, which for S, = 0.1 m/s gives Ck= 1.9 C, and 4.0 C,, respectively. This indi-
cates that, due to gravitational settling, flame propagation through clouds in air of
volatilizing or pyrolyzing particles on the order of 50-100 pm diameter can take place
at considerably lower “static” concentrations C, than for particles of negligible v,.
Burgoyne converted independent experimental data for C, and ck for mists and sprays
of organic liquids to the corresponding ck and C, values, using equation (4.65) and a limit
value of S, of 0.46 m/s for negligible v,estimated by assuming that S, and ck are the same
for upward and downward flame propagation. The results, shown in Figure 4.27, indi-
cate tlhat equation (4.65) is in accordance with reality.
50
a
10
0
0 20 40 60 80 100 120 140 160 180 200
DROP DIAMETER [wml
Figure 4.27 Correlation of ”static” and “kinetic” concentrations at the lower limit of flammability
of tetralidair suspensions with varying drop diameter:
= C, experimental m = C, calculated
o = C,experimental = C, calculated
(From Burgoyne, 1963).