Page 90 - Electrical Properties of Materials
P. 90
72 Bonds
a
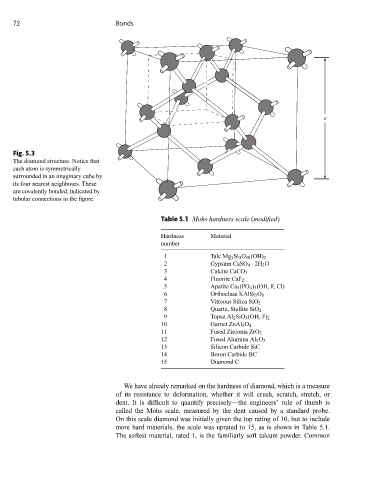
Fig. 5.3
The diamond structure. Notice that
each atom is symmetrically
surrounded in an imaginary cube by
its four nearest neighbours. These
are covalently bonded, indicated by
tubular connections in the figure.
Table 5.1 Mohs hardness scale (modified)
Hardness Material
number
1
Talc Mg Si 4 O 10 (OH) 2
3
2 Gypsum CaSO 4 · 2H 2 O
3 Calcite CaCO 3
4 Fluorite CaF 2
5 Apatite Ca 5 (PO 4 ) 3 (OH, F, Cl)
6 Orthoclase KAlSi 3 O 8
7 Vitreous Silica SiO 2
8 Quartz, Stellite SiO 2
9 Topaz Al 2 SiO 3 (OH, F) 2
10 Garnet ZnAl 2 O 4
11 Fused Zirconia ZrO 2
12 Fused Alumina Al 2 O 3
13 Silicon Carbide SiC
14 Boron Carbide BC
15 Diamond C
We have already remarked on the hardness of diamond, which is a measure
of its resistance to deformation, whether it will crush, scratch, stretch, or
dent. It is difficult to quantify precisely—the engineers’ rule of thumb is
called the Mohs scale, measured by the dent caused by a standard probe.
On this scale diamond was initially given the top rating of 10, but to include
more hard materials, the scale was uprated to 15, as is shown in Table 5.1.
The softest material, rated 1, is the familiarly soft talcum powder. Common