Page 92 - Electrical Properties of Materials
P. 92
74 Bonds
We have described the atoms as consisting of a positive nucleus and the
electrons around the nucleus, with the electrons having certain probabilities of
being in certain places. Since the electrons are sometimes here and sometimes
there, there is no reason why the centres of positive and negative charge should
always be coincident. Thus, we could regard atoms as fluctuating dipoles. If
atom A has a dipole moment, then it will induce an opposite dipole moment
on atom B. On average there will be an attractive force, since the tendency
described leads always to attraction, never to repulsion.
The forces in van der Waals bonds This attraction is called a van der Waals bond. Such bonds are responsible
are fairly weak (and may be shown for the formation of organic crystals.
to vary with the inverse seventh Searching for an anthropomorphic analogy once more (it’s good because
power of distance); consequently it aids the memory) we could look at a dipole as a permanent bond between
these materials have low melting a man and a woman established by mutual attraction. Now would two such
and boiling points. dipoles attract each other? To facilitate the discussion let us introduce the nota-
tion m 1 and m 2 , and w 1 and w 2 for the two men and two women in dipoles 1
and 2 respectively. For an attractive force to develop between two dipoles all
we need is that the attraction between m 1 and w 2 , and m 2 and w 1 should be
stronger than the repulsions between m 1 and m 2 and w 1 and w 2 . In a modern
society this is indeed the likely thing to happen. The attraction can be there
without the need to break the bond.
5.3.5 Mixed bonds
In most practical cases the bonds are of course not any of these pure types. An
example of a mixed bond is that in carbon steel in which the presence of both
metallic and ionic bonds leads to a material with considerably more strength
than that of iron on its own.
Mixed bonds of particular significance to the semiconductor industry are
some III–V and II–VI compounds (where the Roman numbers refer to the
respective columns in the periodic table of Fig. 4.5) as for example GaAs or
ZnSe. They have a combination of ionic and covalent bonds. We shall discuss
their properties in more detail in Section 8.6.
5.3.6 Carbon again
It may be worth noting that the diamond structure is not the only one in which
carbon can crystallize. Another form is graphite, which consists of arrays of
hexagons stuck together in flat sheets. Interestingly, and rather unexpectedly,
lots of further crystalline forms of carbon have been discovered in the last two
decades. Their significance in engineering is not obvious as yet, but they are
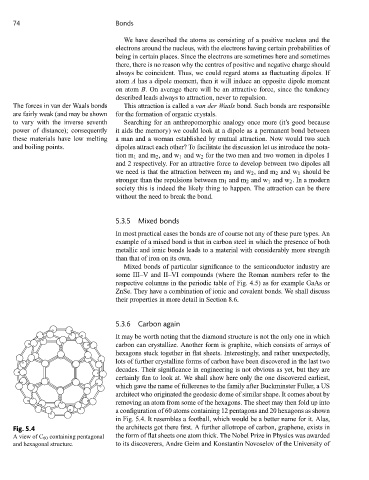
certainly fun to look at. We shall show here only the one discovered earliest,
which gave the name of fullerenes to the family after Buckminster Fuller, a US
architect who originated the geodesic dome of similar shape. It comes about by
removing an atom from some of the hexagons. The sheet may then fold up into
a configuration of 60 atoms containing 12 pentagons and 20 hexagons as shown
in Fig. 5.4. It resembles a football, which would be a better name for it. Alas,
Fig. 5.4 the architects got there first. A further allotrope of carbon, graphene, exists in
AviewofC 60 containing pentagonal the form of flat sheets one atom thick. The Nobel Prize in Physics was awarded
and hexagonal structure. to its discoverers, Andre Geim and Konstantin Novoselov of the University of