Page 96 - Electrical Properties of Materials
P. 96
78 Bonds
If H 12 = H 21 = 0 the two states are not coupled. Then the differential
equation for state (1) is
dw 1
i = H 11 w 1 , (5.27)
dt
which has a solution
H 11
w 1 = K 1 exp –i t . (5.28)
The probability of being in state (1) is thus
2
2
|w 1 (t)| =|K 1 | . (5.29)
This is not a very exciting solution, but it is at least consistent. If there is no
coupling between the states, then the probability of being in state (1) does not
vary with time—once in state (1), always in state (1). The same is true, of
course, for state (2). In the absence of coupling nothing changes.
Before solving the coupled differential equations, let us briefly discuss
the physical concepts of uncoupled states and the meaning of coupling. What
do we mean exactly by coupling? We can explain this with our chosen example,
the hydrogen molecule, or better still the even simpler case, the hydrogen
molecular ion.
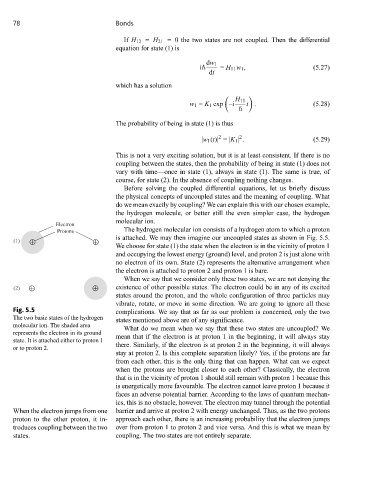
Electron
Protons The hydrogen molecular ion consists of a hydrogen atom to which a proton
⊗ ⊗ We choose for state (1) the state when the electron is in the vicinity of proton 1
(1) is attached. We may then imagine our uncoupled states as shown in Fig. 5.5.
and occupying the lowest energy (ground) level, and proton 2 is just alone with
no electron of its own. State (2) represents the alternative arrangement when
the electron is attached to proton 2 and proton 1 is bare.
When we say that we consider only these two states, we are not denying the
⊗ ⊗ existence of other possible states. The electron could be in any of its excited
(2)
states around the proton, and the whole configuration of three particles may
vibrate, rotate, or move in some direction. We are going to ignore all these
Fig. 5.5 complications. We say that as far as our problem is concerned, only the two
The two basic states of the hydrogen states mentioned above are of any significance.
molecular ion. The shaded area What do we mean when we say that these two states are uncoupled? We
represents the electron in its ground mean that if the electron is at proton 1 in the beginning, it will always stay
state. It is attached either to proton 1
or to proton 2. there. Similarly, if the electron is at proton 2 in the beginning, it will always
stay at proton 2. Is this complete separation likely? Yes, if the protons are far
from each other, this is the only thing that can happen. What can we expect
when the protons are brought closer to each other? Classically, the electron
that is in the vicinity of proton 1 should still remain with proton 1 because this
is energetically more favourable. The electron cannot leave proton 1 because it
faces an adverse potential barrier. According to the laws of quantum mechan-
ics, this is no obstacle, however. The electron may tunnel through the potential
When the electron jumps from one barrier and arrive at proton 2 with energy unchanged. Thus, as the two protons
proton to the other proton, it in- approach each other, there is an increasing probability that the electron jumps
troduces coupling between the two over from proton 1 to proton 2 and vice versa. And this is what we mean by
states. coupling. The two states are not entirely separate.