Page 102 - Bruno Linder Elementary Physical Chemistry
P. 102
August 18, 2010 11:36 9in x 6in b985-ch08 Elementary Physical Chemistry
Introduction to Quantum Theory 87
The Rutherford experiment.
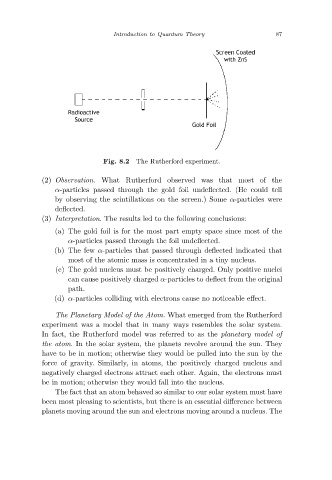
Fig. 8.2
(2) Observation. What Rutherford observed was that most of the
α-particles passed through the gold foil undeflected. (He could tell
by observing the scintillations on the screen.) Some α-particles were
deflected.
(3) Interpretation. The results led to the following conclusions:
(a) The gold foil is for the most part empty space since most of the
α-particles passed through the foil undeflected.
(b) The few α-particles that passed through deflected indicated that
most of the atomic mass is concentrated in a tiny nucleus.
(c) The gold nucleus must be positively charged. Only positive nuclei
can cause positively charged α-particles to deflect from the original
path.
(d) α-particles colliding with electrons cause no noticeable effect.
The Planetary Model of the Atom. What emerged from the Rutherford
experiment was a model that in many ways resembles the solar system.
In fact, the Rutherford model was referred to as the planetary model of
the atom. In the solar system, the planets revolve around the sun. They
have to be in motion; otherwise they would be pulled into the sun by the
force of gravity. Similarly, in atoms, the positively charged nucleus and
negatively charged electrons attract each other. Again, the electrons must
be in motion; otherwise they would fall into the nucleus.
The fact that an atom behaved so similar to our solar system must have
been most pleasing to scientists, but there is an essential difference between
planets moving around the sun and electrons moving around a nucleus. The