Page 103 - Bruno Linder Elementary Physical Chemistry
P. 103
August 18, 2010 11:36 9in x 6in b985-ch08 Elementary Physical Chemistry
88 Elementary Physical Chemistry
sun and planets are neutral (not electrically charged) bodies; the nucleus
and electrons are electrically charged. And, as noted before, when a charged
particle (an electron in this case) moves in an electromagnetic field, it should
emit radiation (light), thereby losing energy. Thus, when an electron moves
around the nucleus it should lose energy and eventually spiral into the
nucleus. In other words, a planetary atom should not exist.
8.4. The Bohr Theory of the Hydrogen Atom
Bohr developed a theoretical model for Rutherford’s atom, using Planck’s
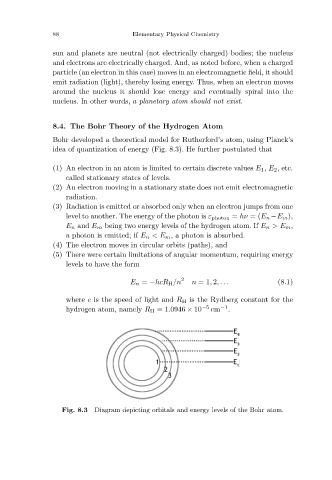
idea of quantization of energy (Fig. 8.3). He further postulated that
(1) An electron in an atom is limited to certain discrete values E 1 , E 2 ,etc.
called stationary states of levels.
(2) An electron moving in a stationary state does not emit electromagnetic
radiation.
(3) Radiation is emitted or absorbed only when an electron jumps from one
level to another. The energy of the photon is ε photon = hν =(E n −E m ),
E n and E m being two energy levels of the hydrogen atom. If E n >E m ,
a photon is emitted; if E n <E m , a photon is absorbed.
(4) The electron moves in circular orbits (paths), and
(5) There were certain limitations of angular momentum, requiring energy
levels to have the form
E n = −hcR H /n 2 n =1, 2,... (8.1)
where c is the speed of light and R H is the Rydberg constant for the
hydrogen atom, namely R H =1.0946 × 10 −5 cm −1 .
Diagram depicting orbitals and energy levels of the Bohr atom.
Fig. 8.3