Page 120 - Bruno Linder Elementary Physical Chemistry
P. 120
August 18, 2010 11:37 9in x 6in b985-ch10 Elementary Physical Chemistry
Quantum Theory. The Chemical Bond 105
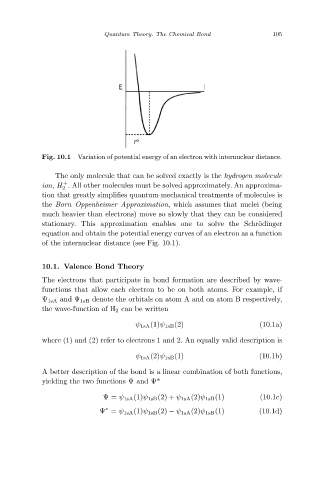
Variation of potential energy of an electron with internuclear distance.
Fig. 10.1
The only molecule that can be solved exactly is the hydrogen molecule
+
ion, H . All other molecules must be solved approximately. An approxima-
2
tion that greatly simplifies quantum-mechanical treatments of molecules is
the Born–Oppenheimer Approximation, which assumes that nuclei (being
much heavier than electrons) move so slowly that they can be considered
stationary. This approximation enables one to solve the Schr¨odinger
equation and obtain the potential energy curves of an electron as a function
of the internuclear distance (see Fig. 10.1).
10.1. Valence Bond Theory
The electrons that participate in bond formation are described by wave-
functions that allow each electron to be on both atoms. For example, if
Ψ 1sA and Ψ 1sB denote the orbitals on atom A and on atom B respectively,
the wave-function of H 2 can be written
ψ 1sA (1)ψ 1sB (2) (10.1a)
where (1) and (2) refer to electrons 1 and 2. An equally valid description is
ψ 1sA (2)ψ 1sB (1) (10.1b)
A better description of the bond is a linear combination of both functions,
yielding the two functions Ψ and Ψ*
Ψ= ψ 1sA(1)ψ 1sB (2) + ψ 1sA (2)ψ 1sB (1) (10.1c)
Ψ = ψ 1sA (1)ψ 1sB (2) − ψ 1sA (2)ψ 1sB (1) (10.1d)
∗