Page 125 - Bruno Linder Elementary Physical Chemistry
P. 125
August 18, 2010 11:37 9in x 6in b985-ch10 Elementary Physical Chemistry
110 Elementary Physical Chemistry
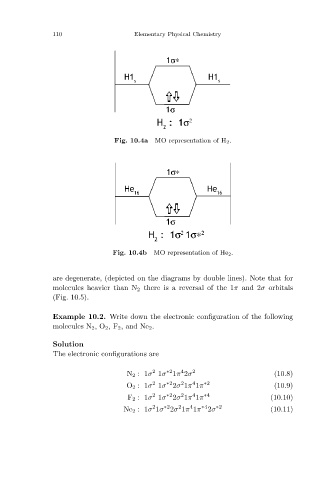
MO representation of H 2.
Fig. 10.4a
MO representation of He 2.
Fig. 10.4b
are degenerate, (depicted on the diagrams by double lines). Note that for
molecules heavier than N 2 there is a reversal of the 1π and 2σ orbitals
(Fig. 10.5).
Example 10.2. Write down the electronic configuration of the following
molecules N 2 ,O 2 ,F 2,and Ne 2.
Solution
The electronic configurations are
2
4
∗2
N 2 :1σ 1σ 1π 2σ 2 (10.8)
4
2
∗2
2
O 2 :1σ 1σ 2σ 1π 1π ∗2 (10.9)
∗2
4
2
2
F 2 :1σ 1σ 2σ 1π 1π ∗4 (10.10)
2
∗4
4
2
∗2
Ne 2 :1σ 1σ 2σ 1π 1π 2σ ∗2 (10.11)