Page 127 - Bruno Linder Elementary Physical Chemistry
P. 127
August 18, 2010 11:37 9in x 6in b985-ch10 Elementary Physical Chemistry
112 Elementary Physical Chemistry
Solution
(1) The bond order of N 2 is 3, as we have seen. The electronic configuration
+ 2 2 4 1
of N is 1σ 1σ* 1π 2σ with bond order of 2.5. Accordingly, N 2 has
2
larger ionization energy.
+ +
(2) The bond order of F 2 is 1 and of F 2 is 3/2. Thus, F 2 has greater
ionization energy.
Homonuclear diatomic molecules are also characterized by parity. This
is a concept that tells whether the wave-function changes sign or remains
unchanged upon reflection through a center of symmetry. The σ orbitals
have even parity (do not change sign) whereas the σ*orbitals have odd
parity (do change sign). The π orbitals have odd parity, whereas the π*
orbitals have even parity. It is common practice to designate the even and
odd parities by the subscripts g and u.Thuswe can write σ g , σ , π u, π .
∗
∗
u g
10.6. Polar Covalent Molecules
Electron pairs between identical atoms are shared equally by the two atoms.
But when the atoms are different, the atoms are pulled closer to one atom
than the other. The ability of an atom to draw electrons close to it is called
electronegativity. Here are electronegativity values of some light elements:
H(2.1), Li(1.01), Be(1.5), B(2.0), C(2.5), N(3.0), O(3.5), F(4.0), Na(0.9).
Why do some atoms pull electrons closer to themselves than others?
The rationale for this may be inferred by analyzing the HF molecule.
The ionization energy of H is 13.6 eV; for F it is 18.8eV. Thus, it would
take about 5 eV more to pull off an electron from F than from H, that is the
energy levels of F and H are not equal; the energy of F is ∼5eV lower. The
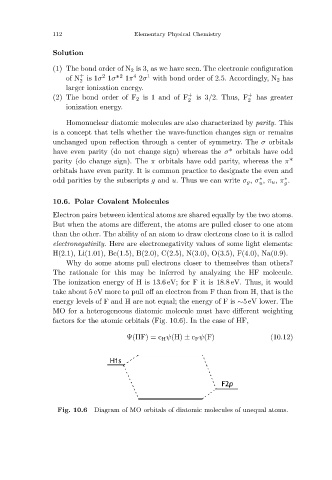
MO for a heterogeneous diatomic molecule must have different weighting
factors for the atomic orbitals (Fig. 10.6). In the case of HF,
Ψ(HF) = c H ψ(H) ± c F ψ(F) (10.12)
Diagram of MO orbitals of diatomic molecules of unequal atoms.
Fig. 10.6