Page 126 - Bruno Linder Elementary Physical Chemistry
P. 126
August 18, 2010 11:37 9in x 6in b985-ch10 Elementary Physical Chemistry
Quantum Theory. The Chemical Bond 111
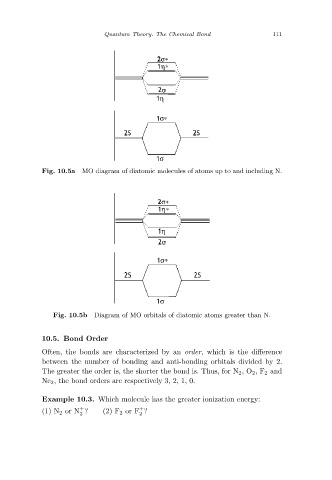
MO diagram of diatomic molecules of atoms up to and including N.
Fig. 10.5a
Diagram of MO orbitals of diatomic atoms greater than N.
Fig. 10.5b
10.5. Bond Order
Often, the bonds are characterized by an order, which is the difference
between the number of bonding and anti-bonding orbitals divided by 2.
The greater the order is, the shorter the bond is. Thus, for N 2 ,O 2,F 2 and
Ne 2 , the bond orders are respectively 3, 2, 1, 0.
Example 10.3. Which molecule has the greater ionization energy:
+
+
(1) N 2 or N ? (2) F 2 or F ?
2 2