Page 41 - Bruno Linder Elementary Physical Chemistry
P. 41
August 18, 2010 11:36 9in x 6in b985-ch03 Elementary Physical Chemistry
26 Elementary Physical Chemistry
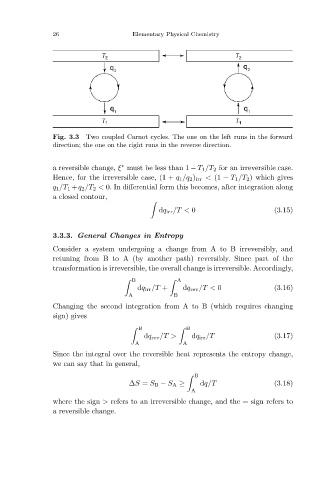
Two coupled Carnot cycles. The one on the left runs in the forward
Fig. 3.3
direction; the one on the right runs in the reverse direction.
∗
a reversible change, ξ must be less than 1 − T 1/T 2 for an irreversible case.
Hence, for the irreversible case, (1 + q 1/q 2) irr < (1 − T 1 /T 2)which gives
q 1 /T 1 +q 2 /T 2 < 0. In differential form this becomes, after integration along
aclosed contour,
dq irr/T < 0 (3.15)
3.3.3. General Changes in Entropy
Consider a system undergoing a change from A to B irreversibly, and
retuning from B to A (by another path) reversibly. Since part of the
transformation is irreversible, the overall change is irreversible. Accordingly,
B A
dq irr /T + dq rev /T < 0 (3.16)
A B
Changing the second integration from A to B (which requires changing
sign) gives
B B
dq rev /T > dq irr /T (3.17)
A A
Since the integral over the reversible heat represents the entropy change,
we can say that in general,
B
∆S = S B − S A ≥ dq/T (3.18)
A
where the sign > refers to an irreversible change, and the = sign refers to
a reversible change.