Page 45 - Bruno Linder Elementary Physical Chemistry
P. 45
August 18, 2010 11:36 9in x 6in b985-ch04 Elementary Physical Chemistry
30 Elementary Physical Chemistry
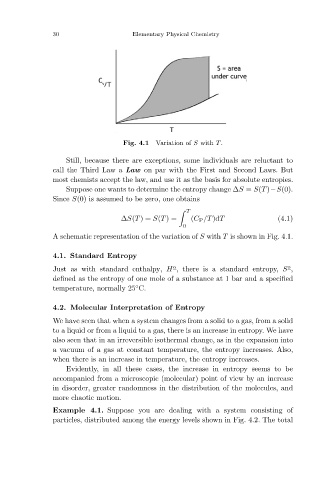
Variation of S with T.
Fig. 4.1
Still, because there are exceptions, some individuals are reluctant to
call the Third Law a Law on par with the First and Second Laws. But
most chemists accept the law, and use it as the basis for absolute entropies.
Suppose one wants to determine the entropy change ∆S = S(T )−S(0).
Since S(0) is assumed to be zero, one obtains
T
∆S(T )= S(T )= (C P /T )dT (4.1)
0
A schematic representation of the variation of S with T is shown in Fig. 4.1.
4.1. Standard Entropy
o
o
Just as with standard enthalpy, H , there is a standard entropy, S ,
defined as the entropy of one mole of a substance at 1 bar and a specified
temperature, normally 25 C.
◦
4.2. Molecular Interpretation of Entropy
We have seen that when a system changes from a solid to a gas, from a solid
to a liquid or from a liquid to a gas, there is an increase in entropy. We have
also seen that in an irreversible isothermal change, as in the expansion into
a vacuum of a gas at constant temperature, the entropy increases. Also,
when there is an increase in temperature, the entropy increases.
Evidently, in all these cases, the increase in entropy seems to be
accompanied from a microscopic (molecular) point of view by an increase
in disorder, greater randomness in the distribution of the molecules, and
more chaotic motion.
Example 4.1. Suppose you are dealing with a system consisting of
particles, distributed among the energy levels shown in Fig. 4.2. The total