Page 46 - Bruno Linder Elementary Physical Chemistry
P. 46
August 18, 2010 11:36 9in x 6in b985-ch04 Elementary Physical Chemistry
The Third Law of Thermodynamics 31
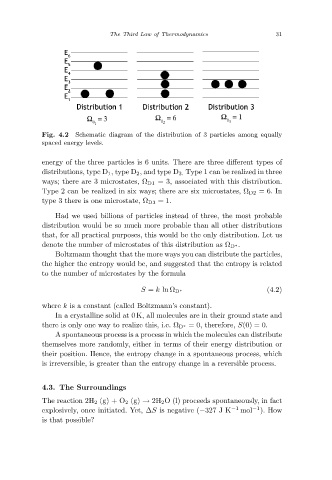
Schematic diagram of the distribution of 3 particles among equally
Fig. 4.2
spaced energy levels.
energy of the three particles is 6 units. There are three different types of
distributions, type D 1,type D 2 ,and type D 3. Type 1 can be realized in three
ways; there are 3 microstates, Ω D1 = 3, associated with this distribution.
Type 2 can be realized in six ways; there are six microstates, Ω D2 =6. In
type 3 there is one microstate, Ω D3 =1.
Had we used billions of particles instead of three, the most probable
distribution would be so much more probable than all other distributions
that, for all practical purposes, this would be the only distribution. Let us
denote the number of microstates of this distribution as Ω D ∗.
Boltzmann thought that the more ways you can distribute the particles,
the higher the entropy would be, and suggested that the entropy is related
to the number of microstates by the formula
(4.2)
S = k ln Ω D ∗
where k is a constant (called Boltzmann’s constant).
In a crystalline solid at 0 K, all molecules are in their ground state and
there is only one way to realize this, i.e. Ω D ∗ = 0, therefore, S(0) = 0.
A spontaneous process is a process in which the molecules can distribute
themselves more randomly, either in terms of their energy distribution or
their position. Hence, the entropy change in a spontaneous process, which
is irreversible, is greater than the entropy change in a reversible process.
4.3. The Surroundings
The reaction 2H 2 (g) + O 2 (g) → 2H 2 O (l) proceeds spontaneously, in fact
explosively, once initiated. Yet, ∆S is negative (−327 J K −1 mol −1 ). How
is that possible?