Page 145 - Elements of Chemical Reaction Engineering 3rd Edition
P. 145
Chap. 3 Questions and Problems 117
The initial concentrations of ethylene oxide and water are 1 lb mol/ft3
and 3.47 Ib-mol/ft3 (62.41 lb/ft3 + 18), respectively.
(b) The isothermal, isobaric gas-phase pyrolysis
C,H6 __j C2H4 + H2
Pure ethane enters the flow reactor at 6 atm and 1100 K.
HOW would your equation for the concentration change if the reaction
were to be carried out in a constant-volume batch reactor?
(c) The icothermal, isobaric, catalytic gas-phase oxidation
/"\
C,H, + 0, -----+ CH,-CH2
The feed enters a! PBR at 6 atm and 260°C and is a stoichiometric mix-
ture of oxygen and ethylene.
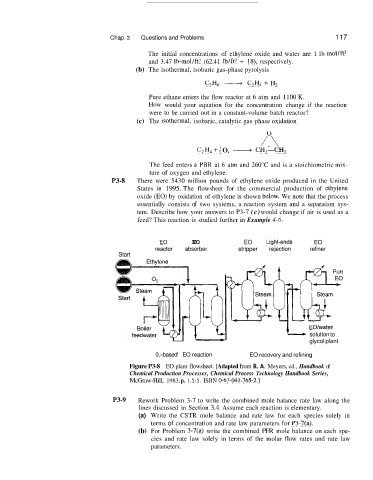
P3-8 There were 5430 million pounds of ethylene oxide produced in the United
States in 1995. The flowsheet for the commercial production of ethlylene
oxide (EO) by oxidation of ethylene is shown blzlow. We note that the process
essentially consists of two systems, a reaction system and a separation sys-
tem. Describe how your answers to P3-7 (c) would change if air is used as a
feed? This reaction is studied further in Example 4-6.
EO EO EO 1-ight-ends EO
reactor absorber stripper rejection refiner
Start
EOJwater
solution to
glycol plant
0,--based' EO reaction EO recovery and refining
Figure P3-8 EO plant flowsheet. [Adapted from R. A. Meyers, ed., Handbook of
Chemical Production Processes, Chemical Process Technology Handbook Series,
McGraw-Hill, 1983, p. 1.5-5. ISBN 0-67-041-765-2.1
P3-9 Rework Problem 3-7 to write the combined mole balance rate law along the
lines discussed in Section 3.4. Assume each reaction is elementary.
(a) Write the CSTR mole balance and rate law for each species solely in
terms of concentration and rate law parameters for P3-7(a).
(b) For Problem 3-7(a) write the combined PFR mole balance on each spe-
cies and rate law solely in terms of the molar flow rates and rate law
parameters.