Page 213 - Elements of Chemical Reaction Engineering 3rd Edition
P. 213
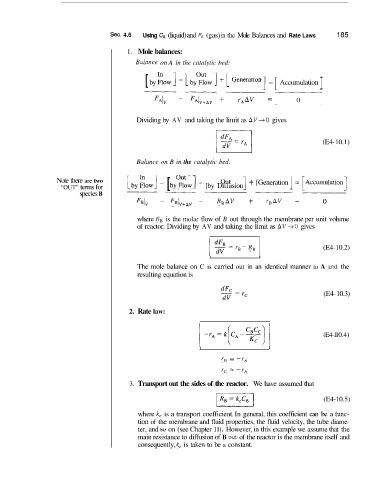
Sec. 4.6 Using CA (liquid) and fA (gas) in the Mole Balances and Rate Laws 185
1. Mole balances:
Bulance on A in the catalytic bed:
I
[ by :ow ] - [ by%& ] + [ Generation ] = [ Accumulation
Dividing by AV and taking the limit as AV+O gives
(E4- 10.1)
Balance on B in the catalytic bed:
Note there are two ] + [Generation
Out
“OUT” terms for [by :OW ] - [ by(%~W] - [by Diffusion
species B
FB~~ FB(~+~~ RBAV + r,AV = 0
-
-
where RB is the molar flow of B out through the membrane per unit volume
of reactor. Dividing by AV and taking the limit as AV+O gives
I 2 =rB-RB (E4-10.2)
I I
The mole balance on C is carried out in an identical manner to A and the
resulting equation is
(E4- 10.3)
2. Rate law:
(E4-II 0.4)
I J
r, = -r,
r, = -rA
3. Transport out the sides of the reactor. We have assumed that
(E4-10.5)
where k, is a transport coefficient. In general, this coefficient can be a fiunc-
tion of the membrane and fluid properties, the fluid velocity, the tube diame-
ter, and so on (see Chapter 11). However, in this example we assume that the
main resistance to diffusion of B out of the reactor is the membrane itself and
consequently, k, is taken to be a constant.