Page 209 - Elements of Chemical Reaction Engineering 3rd Edition
P. 209
SW. 4.6 Using CA (liquid) and FA (gas) in the Mole Balances and Rate Laws 181
KEY! f
20.000
-- 16.000
- fa
--fb _..*- _...-- e.
12.000 -- d., ,... e .. _,.--
8.000 :\\ .#I*' /' $2' ..'
4.000 -- ,!
,*
0.000 , ' I I I I I I I I I I I !-I
!-I
I
0.000
oc
20.000
0.000
40.000
100.
80.000
60.000
0.000 20.000 40.000 60.000 80.000 100. oc
V
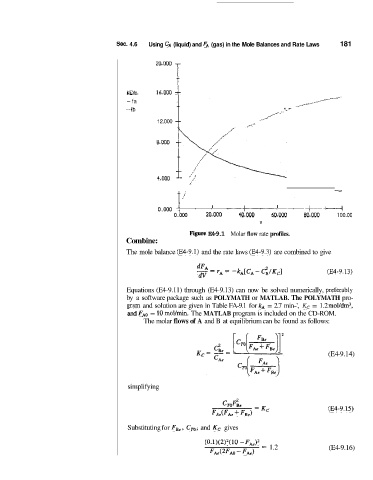
Fipre E4-9.1 Molar flow rate profiles.
Combine:
The mole balance ($4-9.1) and the rate laws (E4-9.3) are combined to give
(E4-9.13)
Equations (E4-9.11) through (E4-9.13) can now be solved numerically, prefierably
by a software package such as POLYMATH or MATLAB. The POLYMATH pro-
gr,m and solution are given in Table FA-9.1 for kA = 2.7 min-', Kc = 1.2 m01/dm3,
and FA0 = 10 moYmin. The MATLAB program is included on the CD-ROM.
The molar flows of A and B at equilibrium can be found as follows:
(E4-9.14)
simplifying
(E4-9.15)
Substituting for FBe, CTo, and K, gives
(0.1)(2)2( 10 - FAe)2
= 1.2 (E4-9.16)
FA~(2FA0 - FAe)