Page 222 - Elements of Chemical Reaction Engineering 3rd Edition
P. 222
194 Isothermal Reactor Design Chap. 4
I o.ooo20/-
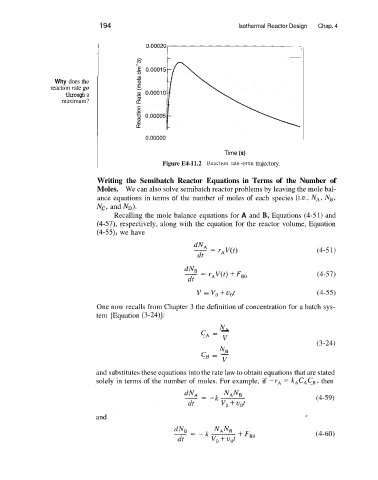
Why does the
reaction rate go
through a
maximum?
0.00000
Time (s)
Figure E4-11.2 Reactlon rate-tlme trajectory.
Writing the Semibatch Reactor Equations in Terms of the Number of
Moles. We can also solve semibatch reactor problems by leaving the mole bal-
ance equations in terms of the number of moles of each species (Le., NA, N,,
N,, and N~).
Recalling the mole balance equations for A and B, Equations (4-5 1) and
(4-57), respectively, along with the equation for the reactor volume, Equation
(4-55), we have
(4-5 1)
(4-57)
v = V,+v,t (4-55)
One now recalls from Chapter 3 the definition of concentration for a batch sys-
tem [Equation (3-24)]:
c, = -
N.4
V
(3-24)
NB
c, = -
V
and substitutes these equations into the rate law to obtain equations that are stated
solely in terms of the number of moles. For example, if -rA = kAC,C,, then
(4-59)
and
(4-60)