Page 224 - Elements of Chemical Reaction Engineering 3rd Edition
P. 224
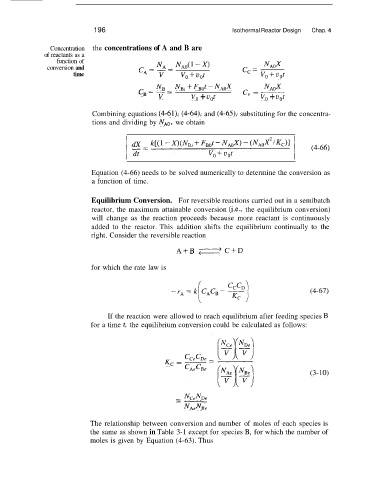
196 Isothermal Reactor Design Chap. 4
Concentration the concentrations of A and B are
of reactants as a
function of
conversion and
time
CB = - + NAOx
NB = N%~ FBOt - NAOX
V v, + UOt c, = ~ v, + UOt
Combining equations (4-611, (4-64), and (4-65), substituting for the concentra-
tions and dividing by NAo, we obtain
Equation (4-66) needs to be solved numerically to determine the conversion as
a function of time.
Equilibrium Conversion. For reversible reactions carried out in a semibatch
reactor, the maximum attainable conversion (i.e., the equilibrium conversion)
will change as the reaction proceeds because more reactant is continuously
added to the reactor. This addition shifts the equilibrium continually to the
right. Consider the reversible reaction
ASB e C + D
for which the rate law is
(4-67)
If the reaction were allowed to reach equilibrium after feeding species B
for a time t, the equilibrium conversion could be calculated as follows:
K, =
(3-10)
--
- NCeND,
NAeNBe
The relationship between conversion and number of moles of each species is
the same as shown in Table 3-1 except for species B, for which the number of
moles is given by Equation (4-63). Thus