Page 217 - Elements of Chemical Reaction Engineering Ebook
P. 217
188 Isothermal Reactor Design Chap. 4
time necessary to reach steady-state operation (see Figure 4-13a). Next, semi- '
batch reactors are discussed. In each of these cases, we are interested in pre-
dicting the concentration and conversion as a function of time. Closed-form
. analytical solutions to the differential equations arising from the mole balance
of these reaction types can be obtained only for zero- and first-order reactions.
CAo-m
ODE solvers must be used for other orders.
-
I
CA
(b) (C)
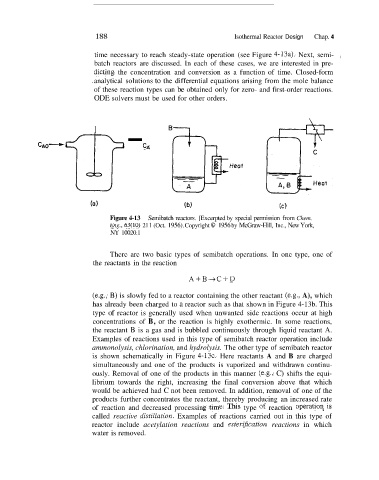
Figure 4-13 Semibatch reactors. [Excerpted by special permission from Chem.
Eng., 63(10) 21 1 (Oct. 1956). Copyright 0 1956 by McGraw-Hill, Inc., New York,
NY 10020.1
There are two basic types of semibatch operations. In one type, one of
the reactants in the reaction
A+B-+C+D
(e.g., B) is slowly fed to a reactor containing the other reactant (e.g., A), which
has already been charged to a reactor such as that shown in Figure 4-13b. This
type of reactor is generally used when unwanted side reactions occur at high
concentrations of B, or the reaction is highly exothermic. In some reactions,
the reactant B is a gas and is bubbled continuously through liquid reactant A.
Examples of reactions used in this type of semibatch reactor operation include
ammonolysis, chlorination, and hydrolysis. The other type of semibatch reactor
is shown schematically in Figure 4-13c. Here reactants A and B are charged
simultaneously and one of the products is vaporized and withdrawn continu-
ously. Removal of one of the products in this manner (e.g., C) shifts the equi-
librium towards the right, increasing the final conversion above that which
would be achieved had C not been removed. In addition, removal of one of the
products further concentrates the reactant, thereby producing an increased rate
of reaction and decreased processin e. This type of reaction operatioq is
called reactive distilzation. Examples of reactions carried out in this type of
reactor include acetylation reactions and esteriJication reactions in which
water is removed.