Page 339 - Elements of Chemical Reaction Engineering Ebook
P. 339
31 0 Multiple Reactions Chap. 6
TO var~ TCSTR
one can vary
either vo for a
fixed Vor varyV
for a fixed vo C
.-
0
c
0.5
0.0 1 .o 2.0
‘CSTR
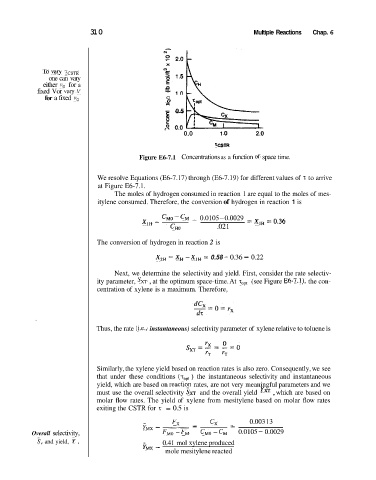
Figure E6-7.1 Concentrations as a function of space time.
We resolve Equations (E6-7.17) through (E6-7.19) for different values of z to arrive
at Figure E6-7.1.
The moles of hydrogen consumed in reaction 1 are equal to the moles of mes-
itylene consumed. Therefore, the conversion of hydrogen in reaction 1 is
CMO-~M 0.0105-0.0029 = *,, = o.36
-
xlH = c,, - .02 1
’
The conversion of hydrogen in reaction 2 is
X,, X, - X,, 0.58 - 0.36 = 0.22
Next, we determine the selectivity and yield. First, consider the rate selectiv-
ity parameter, SxT , at the optimum space-time. At zopt (see Figure E6-7.1), the con-
centration of xylene is a maximum. Therefore,
Thus, the rate (i.e., instantaneous) selectivity parameter of xylene relative to toluene is
Similarly, the xylene yield based on reaction rates is also zero. Consequently, we see
that under these conditions ( zOpt ) the instantaneous selectivity and instantaneous
yield, which are based on reactio? rates, are not very meaningful parameters and we
must use the overall selectivity SXT and the overall yield YXT , which are based on
molar flow rates. The yield of xylene from mesitylene based on molar flow rates
exiting the CSTR for ‘c = 0.5 is
- Fx = cx = 0.003 13
YMX = -
Overall selectivity, F,, - FM CMo - CM 0.0105 - 0.0029
s, and yield, ? . - 0.41 mol xylene produced
yMx = mole mesitylene reacted