Page 416 - Elements of Chemical Reaction Engineering Ebook
P. 416
Sec. 7.4 Enzymatic Reaction Fundamentals 387
Dividing the numerator and denominator of Equation (7-86) by k, , we obtain
a form of the Michaelis-Menten equation:
(7-87)
where K, is called the Michaelis constant. If, in addition, we let V,, represent
the maximum rate of reaction for a given total enzyme concentration,
V,, = k;(E,) (7-88)
the Michaelis-Menten equation takes the familiar form
r I
Michaelis-Menten (7-89)
equation
I
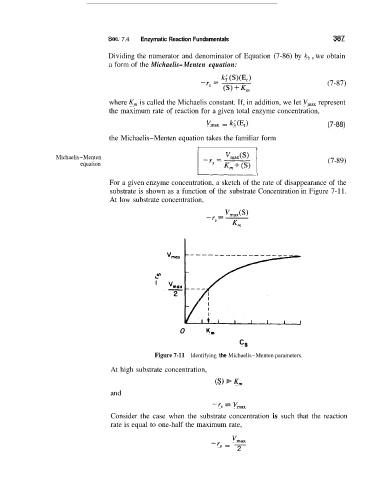
For a given enzyme concentration, a sketch of the rate of disappearance of the
substrate is shown as a function of the substrate Concentration in Figure 7-11.
At low substrate concentration,
0
CS
Figure 7-11 Identifying the Michaelis-Menten parameters.
At high substrate concentration,
(9 K,,,
and
-r, GZ V,,
Consider the case when the substrate concentration is such that the reaction
rate is equal to one-half the maximum rate,
"ma,
-rs = -
2