Page 420 - Elements of Chemical Reaction Engineering Ebook
P. 420
Sec. 7.4 Enzymatic Reaction Fundamentals 39 1
-
0
so- s
t
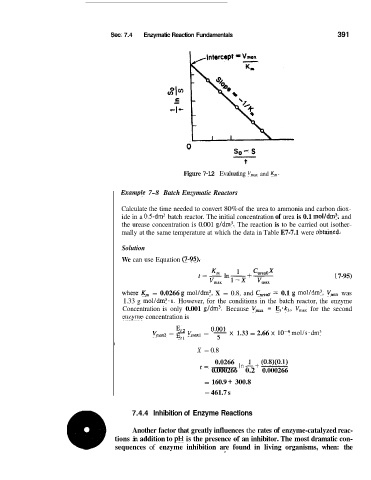
Figure 7-12 Evaluating V,, and K,.
Exczmple 7-8 Batch Enzymatic Reactors
Calculate the time needed to convert 80% of the urea to ammonia and carbon diox-
ide in a 0.5-dm3 batch reactor. The initial concentration of urea is 0.1 mol/dm3, and
the urease concentration is 0.001 g/dm3. The reaction is to be carried out isother-
mally at the same temperature at which the data in Table E7-7.1 were obtaine:d.
Solution
We can use Equation (7-95),
r: 7-95)
whiere K, = 0.0266 g mol/dm3, X = 0.8, and CuEaO = 0.1 g mol/dm3, V,,, was
1.33 g mol/dm3.s. However, for the conditions in the batch reactor, the enzyme
Concentration is only 0.001 g/dm3. Because V,, = E,.k3, V,,, for the second
enz,yme concentration is
vm,z = - v,,, = - mol/s.dm3
E* 2
o'ool X 1.33 = 2.66 X
Et 1 5
I
X = 0.8
t= -
1
0.0266
(0.8)(0.1)
ln-+
0.000266 0.2 0.000266
= 160.9 -t 300.8
= 461.7 s
7.4.4 Inhibition of Enzyme Reactions
Another factor that greatly influences the rates of enzyme-catalyzed reac-
tions in addition to pH is the presence of an inhibitor. The most dramatic con-
sequences of enzyme inhibition are found in living organisms, when: the
I