Page 457 - Elements of Chemical Reaction Engineering Ebook
P. 457
428 Steady-State Nonisothermal Reactor Design Chap. 8
crosses the system boundaries, the change in total energy of the system, dk,
is equal to the heat flow to the system, SQ, minus the work done by the system
on the surroundings, SW For a closed system, the energy balance is
The 6's signify that SQ and SW are not exact differentials of a state function.
The continuous-flow reactors we have been discussing are considered to
be open systems in that mass crosses the system boundary. We shall carry put
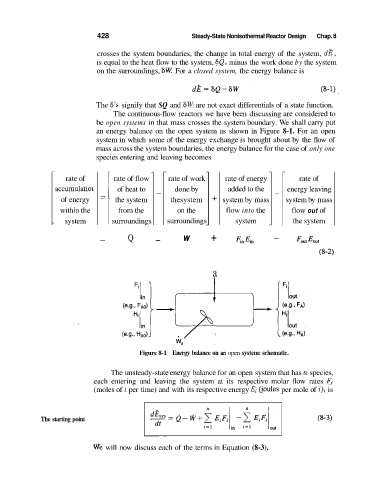
an energy balance on the open system as shown in Figure 8-1. For an open
system in which some of the energy exchange is brought about by the flow of
mass across the system boundaries, the energy balance for the case of only one
species entering and leaving becomes
- - - - - -
rate of rate of flow rate of work rate of energy rate of
accumulatioi of heat to done by added to the energy leaving
of energy the system - thesystem , + system by mass - system by mass
within the from the on the flow into the flow out of
- system surroundings surroundings - system - the system
- - Q - w +
a
Figure 8-1 Energy balance on an open system: schematic.
The unsteady-state energy balance for an open system that has n species,
each entering and leaving the system at its respective molar flow rates Fi
(moles of i per time) and with its respective energy Ei Cjoules per mole of i), is
The starting point (8-3)
~~
we will now discuss each of the terms in Equation (8-3).