Page 462 - Elements of Chemical Reaction Engineering Ebook
P. 462
Sec. 8.2 The Energy Balance 433
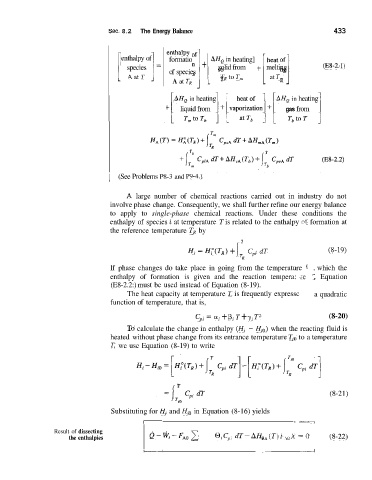
[ AatT 1- formation '1 + rHQ in heating] + [ heat of] (E8-2. I)
enthalpy of
solid from
melting
species
-
of species
A at T, TR to T, at T,
AHQ in heating
AHQ in heating + 1 ~ gas from 1
1 liquid from 1 + [ ~ a ~ ~ z ~ ~ o ~
1 (See Problems P8-3 and €94.)
A large number of chemical reactions carried out in industry do not
involve phase change. Consequently, we shall further refine our energy balance
to apply to single-phase chemical reactions. Under these conditions the
enthalpy of species i at temperature T is related to the enthalpy cf formation at
the reference temperature TR by
Hi = H;(T,) + Cpi dT (8-19)
If phase changes do take place in going from the temperature F . which the
enthalpy of formation is given and the reaction tempera: .re Equation
(E8-2.2:) must be used instead of Equation (8-19).
The heat capacity at temperature T is frequently expressc a quadratic
function of temperature, that is,
Cpl = a, + p, T + y, T2 (8-20)
$3 calculate the change in enthalpy (H, - Hio) when the reacting fluid is
heated without phase change from its entrance temperature Tz0 to a temperature
T, we use Equation (8-19) to write
T
(8-21)
Substituting for Hi and Hi, in Equation (8-16) yields
.. I
1
Result of dissecting
the enthalpies
I .- 2