Page 470 - Elements of Chemical Reaction Engineering Ebook
P. 470
Sec. 8.3 Nonisothermal Continuous-Flow Reactors 44 1
How can we use this information? Let's stop a minute and consider a sys-
tem with the special set of conditions of no work, Ws = 0, adiabatic operation
Q = 0, and then rearrange (8-48) into the form
(8-49)
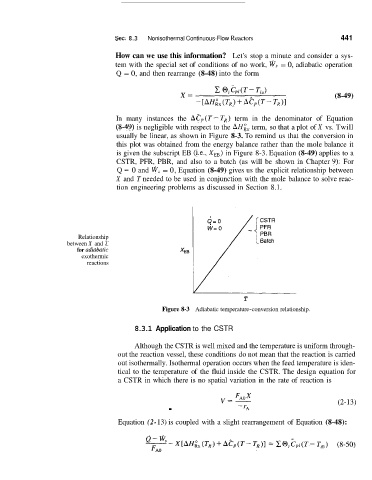
In many instances the Aep(T--TR) term in the denominator of Equation
(8-49) is negligible with respect to the AH:, term, so that a plot of X vs. Twill
usually be linear, as shown in Figure 8-3. To remind us that the conversion in
this plot was obtained from the energy balance rather than the mole balance it
is given the subscript EB (Le., XEB) in Figure 8-3. Equation (8-49) applies to a
CSTR, PFR, PBR, and also to a batch (as will be shown in Chapter 9). For
Q = 0 and Ws = 0, Equation (8-49) gives us the explicit relationship between
X and T needed to be used in conjunction with the mole balance to solve reac-
tion engineering problems as discussed in Section 8.1.
Relationship
between X and T
for adiabaric
exothermic
reactions
T
Figure 8-3 Adiabatic temperature-conversion relationship.
8.3.1 Application to the CSTR
Although the CSTR is well mixed and the ternperature is uniform through-
out the reaction vessel, these conditions do not mean that the reaction is carried
out isothermally. Isothermal operation occurs when the feed temperature is iden-
tical to the temperature of the fluid inside the CSTR. The design equation for
a CSTR in which there is no spatial variation in the rate of reaction is
. (2-13)
Equation (2- 13) is coupled with a slight rearrangement of Equation (8-48):