Page 475 - Elements of Chemical Reaction Engineering Ebook
P. 475
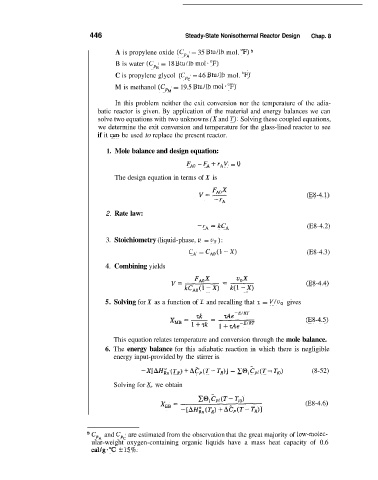
446 Steady-State Nonisothermal Reactor Design Chap. 8
A is propylene oxide (CpA = 35 Btu/lb mol. OF)
B is water (Cp, = 18 Btu/lb mol-"F)
C is propylene glycol ( Cp, = 46 Btu/lb mol. OF)
M is methanol (CpM = 19.5 Btu/lb mol. OF)
In this problem neither the exit conversion nor the temperature of the adia-
batic reactor is given. By application of the material and energy balances we can
solve two equations with two unknowns (X and T). Solving these coupled equations,
we determine the exit conversion and temperature for the glass-lined reactor to see
if it can be used to replace the present reactor.
1. Mole balance and design equation:
FAo - FA i- rAV = 0
The design equation in terms of X is
(E8-4.1)
2. Rate law:
-rA = kCA (E8-4.2)
3. Stoichiometry (liquid-phase, u = uo ):
CA = CA0(l -X) (E8-4.3)
4. Combining yields
(E8-4.4)
5. Solving for X as a function of T and recalling that 7 = V/uo gives
(E8-4.5)
This equation relates temperature and conversion through the mole balance.
6. The energy balance for this adiabatic reaction in which there is negligible
energy input-provided by the stirrer is
-X[AHg (TK) i- Aep (T- TR)] = CO, Z7p1 (T- TO) (8-52)
Solving for X, we obtain
(E8-4.6)
CpA and Cp, are estimated from the observation that the great majority of low-molec-
ular-weight oxygen-containing organic liquids have a mass heat capacity of 0.6
d/g-"C +-15%.