Page 478 - Elements of Chemical Reaction Engineering Ebook
P. 478
Sec. 8.3 Noniscithermal Continuous-Flow Reactors 449
I.Or
The reactor cannot
be used because it
will exceed the
specified maximum
temperature
of 585 R
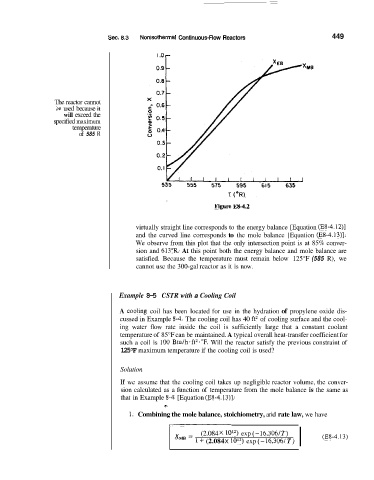
Figure ES-4.2
virtually straight line corresponds to the energy balance [Equation (E8-4.12)]
and the curved line corresponds to the mole balance [Equation (E8-4.13)].
We observe from this plot that the only intersection point is at 85% conver-
sion and 613"R. At this point both the energy balance and mole balance are
satisfied. Because the temperature must remain below 125°F (585 R), we
cannot use the 300-gal reactor as it is now.
Example 8-5 CSTR with a Cooling Coil
A coioling coil has been located for use in the hydration of propylene oxide dis-
cussed in Example 8-4. The cooling coil has 40 ft2 of cooling surface and the cool-
ing water flow rate inside the coil is sufficiently large that a constant coolant
temperature of 85°F can be maintained. A typical overall heat-transfer coefficient for
such a coil is 100 Btu/h*ft*."F. Will the reactor satisfy the previous constraint of
125°F maximum temperature if the cooling coil is used?
Solution
If we assume that the cooling coil takes up negligible reactor volume, the conver-
sion calculated as a function of temperature from the mole balance is the same as
that in Example 8-4 [Equation (E8-4.13)].
1c
1. Combining the mole balance, stoichiometry, arid rate law, we have
(2.084 X lo1*) exp (- 16,306/T) I
XMB = 1 i- (2.084 X loL2) exp (- 16,306/ T) (ES-4.13)