Page 487 - Elements of Chemical Reaction Engineering Ebook
P. 487
458 Steady-State Nonisothermal Reactor Design Chap. 8
Let's calculate the CSTR volume necessary to achieve 40% conversion. The
mole balance is
Using Equation (E8-6.2) in the mole balance, we obtain
V= , FAOX (E8-6.16)
From the energy balance we have Equation (E8-6.10):
T = 330 + 43.3X
= 330 + 43.3(0.4) = 347.3
Using Equations (ES-6.11) and (E8-6.12) or from Table E8-6.1,
k = 13.93 h-l
K, = 2.73
Then
-rA = 58.6 kmol/m3. h
The adiabatic
CSTR volume is less b' = ( 146.7 kmol butane/ h) (0.4)
than the PFR 58.6 kmol/m3 * h
volume
v = 1.0 m3
We see that the CSTR volume (1 m3) to achieve 40% conversion in this adiabatic
reaction is less than the PFR volume (1.15 m3).
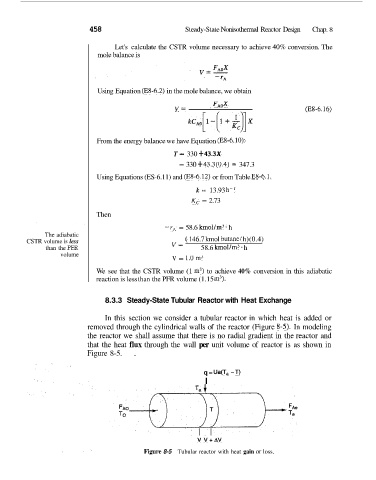
8.3.3 Steady-State Tubular Reactor with Heat Exchange
In this section we consider a tubular reactor in which heat is added or
removed through the cylindrical walls of the reactor (Figure 8-5). In modeling
the reactor we shall assume that there is no radial gradient in the reactor and
that the heat flux through the wall per unit volume of reactor is as shown in
Figure 8-5. .
q = Ua(T, - T)
I
VV+AV
Figure 8-5 Tubular reactor with heat gain or loss.