Page 490 - Elements of Chemical Reaction Engineering Ebook
P. 490
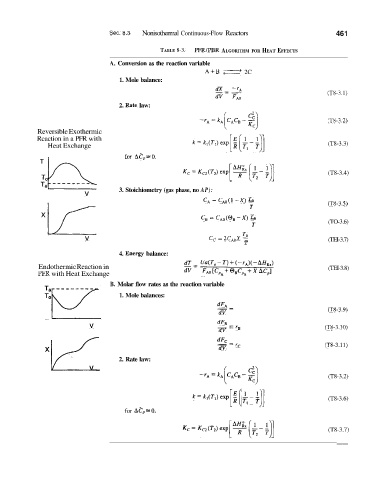
Sec. 8.3 Nonisothermal Continuous-Flow Reactors 46 1
TABLE 8-3. PFR/PBR ALGORITHM FOR HEAT EFFECTS
A. Conversion as the reaction variable
A+B 2C
1. Mole balance:
(T13-3.1)
2. R.ate law:
(T8-3.2)
Reversible Exothermic
Reaction in a PFR with
Heat Exchange (T8-3.3)
for Aep=O.
(T8-3.4)
v 3. Stoichiometry (gas phase, no AP):
CA = CAo(1 -X) -
TO
T (T8-3.5)
TO
CB = cAo(@B-X) -
T (TO-3.6)
TO
v c, = 2c,,x - (TEI-3.7)
T
4. Einergy balance:
Endothermic Reaction in (TEI-3.8)
PFR with Heat Exchange
B. Molar flow rates as the reaction variable
1. Mole balances:
dFA -
--
dV (T8'-3.9)
v dFu - rB (T8-3.10)
--
dV
xE -- rc (T8-.3.11)
dFc -
dV
2. Rate law:
v
(T8-3.2)
["( '11
k=k,(T,)exp E T;-T (T8-3.6)
for AZ'p=O.
(T8-3.7)
-