Page 495 - Elements of Chemical Reaction Engineering Ebook
P. 495
466 Steady-State Nonisothermal Reactor Design Chap. 8
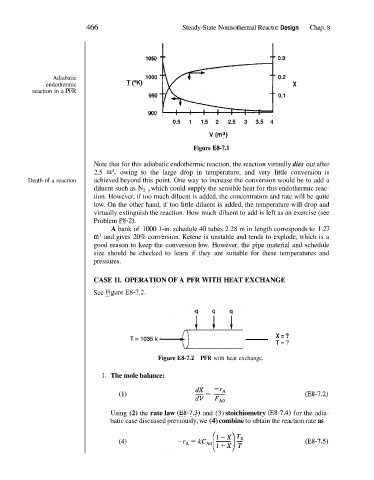
1050 I 1
0.3
Adiabatic 1000 0.2
endothermic T (OK) X
reaction in a PFR
950 0.1
900
0.5 1 1.5 2 2.5 3 3.5 4
v (m3)
Figure E8-7.1
Note that for this adiabatic endothermic reaction, the reaction virtually dies out after
2.5 m3, owing to the large drop in temperature, and very little conversion is
Death of a reaction achieved beyond this point. One way to increase the conversion would be to add a
diluent such as N, , which could supply the sensible heat for this endothermic reac-
tion. However, if too much diluent is added, the concentration and rate will be quite
low. On the other hand, if too little diluent is added, the temperature will drop and
virtually extinguish the reaction. How much diluent to add is left as an exercise (see
Problem P8-2).
A bank of 1000 1-in. schedule 40 tubes 2.28 m in length corresponds to 1.27
m3 and gives 20% conversion. Ketene is unstable and tends to explode, which is a
good reason to keep the conversion low. However, the pipe material and schedule
size should be checked to learn if they are suitable for these temperatures and
pressures.
CASE 11. OPERATION OF A PFR WITH HEAT EXCHANGE
See Figdre E8-7.2.
X=?
T=?
Figure E8-7.2 PFR with heat exchange.
1. The mole balance:
(E8-7.2)
Using (2) the rate law (E8-7.3) and (3) stoichiometry (E8-7.4) for the adia-
batic case discussed previously, we (4) combine to obtain the reaction rate as
(E8-7.5)