Page 497 - Elements of Chemical Reaction Engineering Ebook
P. 497
468 Steady-State Nonisothermal Reactor Design Chap. 8
PFR with heat
exchange
VX103 (dd)
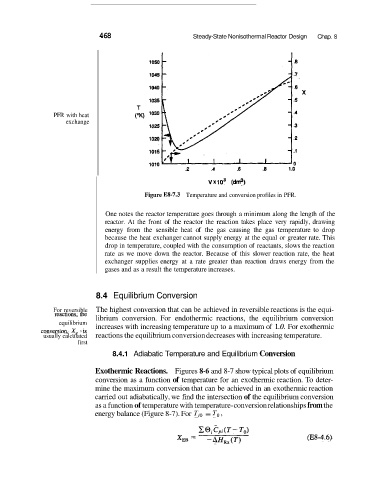
Figure E8-7.3 Temperature and conversion profiles in PFR.
One notes the reactor temperature goes through a minimum along the length of the
reactor. At the front of the reactor the reaction takes place very rapidly, drawing
energy from the sensible heat of the gas causing the gas temperature to drop
because the heat exchanger cannot supply energy at the equal or greater rate. This
drop in temperature, coupled with the consumption of reactants, slows the reaction
rate as we move down the reactor. Because of this slower reaction rate, the heat
exchanger supplies energy at a rate greater than reaction draws energy from the
gases and as a result the temperature increases.
8.4 Equilibrium Conversion
For reversible The highest conversion that can be achieved in reversible reactions is the equi-
reactions, *e librium conversion. For endothermic reactions, the equilibrium conversion
equilibrium
conversion, X, , is increases with increasing temperature up to a maximum of 1 .O. For exothermic
usually calculated reactions the equilibrium conversion decreases with increasing temperature.
first
8.4.1 Adiabatic Temperature and Equilibrium Conversion
Exothermic Reactions. Figures 8-6 and 8-7 show typical plots of equilibrium
conversion as a function of temperature for an exothermic reaction. To deter-
mine the maximum conversion that can be achieved in an exothermic reaction
carried out adiabatically, we find the intersection of the equilibrium conversion
as a function of temperature with temperature-conversion relationships from the
energy balance (Figure 8-7). For T,, = To,
(E8-4.6)