Page 498 - Elements of Chemical Reaction Engineering Ebook
P. 498
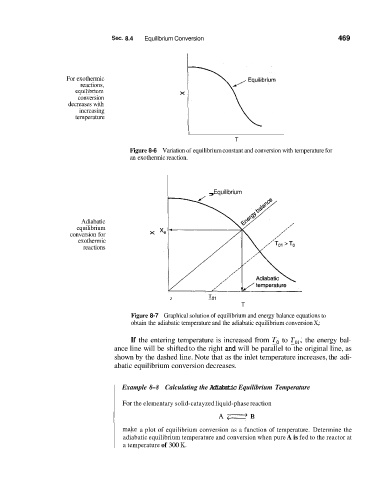
Sec. 8.4 Equilibrium Conversion 469
For exothermic
reactions,
equilibriem
conversion
decreases with
increasing
temperature
I
T
Figure 8-6 Variation of equilibrium constant and conversion with temperature for
an exothermic reaction.
, Equilibrium
Adiabatic
equilibrium
conversion for
exothermic
reactions
I To1
T
Figure 8-7 Graphical solution of equilibrium and energy balance equations to
obtain the adiabatic temperature and the adiabatic equilibrium conversion X,:
If the entering temperature is increased from To to Tal, the energy bal-
ance line will be shifted to the right and will be parallel to the original line, as
shown by the dashed line. Note that as the inlet temperature increases, the adi-
abatic equilibrium conversion decreases.
Example 8-8 Calculating the Adiabatic Equilibrium Temperature
For the elementary solid-catayzed liquid-phase reaction
mak.e a plot of equilibrium conversion as a function of temperature. Determine the
adiabatic equilibrium temperature and conversion when pure A is fed to the reactor at
a temperature of 300 K.