Page 502 - Elements of Chemical Reaction Engineering Ebook
P. 502
Sec. 8.4 Equilibrium Conversion 473
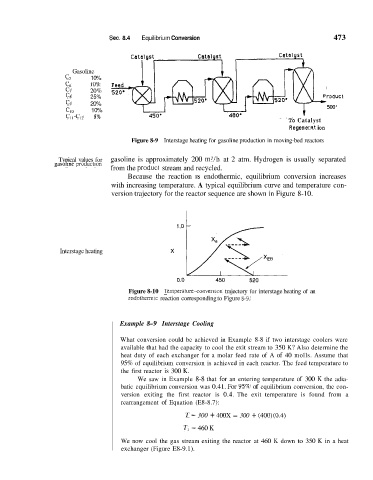
Gasoline
c5 10%
Feed
10%
cb 20% 520.
c7
CS 25% roduct
c9 20% 500'
Cl0 10%
cII-cI2 5%
To Catalyst
Re ge n e r a~t i on
Figure 8-9 Interstage heating for gasoline production in moving-bed reactors
Typical values for gasoline is approximately 200 m3/h at 2 atm. Hydrogen is usually separated
gasollne production from the producl stream and recycled.
Because the reaction 1s endothermic, equilibrium conversion increases
with increasing temperature. A typical equilibrium curve and temperature con-
version trajectory for the reactor sequence are shown in Figure 8-10.
Interstage heating
Figure 8-10 Temperaturesonversion trajectory for interstage heating of an
eridothermic reaction corresponding to Figure 8-9.
Example 8-9 Interstage Cooling
What conversion could be achieved in Example 8-8 if two interstage coolers were
available that had the capacity to cool the exit stream to 350 K? Also determine the
heat duty of each exchanger for a molar feed rate of A of 40 molls. Assume that
95% of equilibrium conversion is achieved in each reactor. The feed temperature to
the first reactor is 300 K.
We saw in Example 8-8 that for an entering temperature of 300 K the adia-
batic equilibrium conversion was 0.41. For 95% of equilibrium conversion, the con-
version exiting the first reactor is 0.4. The exit temperature is found from a
rearrangement of Equation (E8-8.7):
T = 300 + 400X = 300 + (400)(0.4)
T, = 460 K
We now cool the gas stream exiting the reactor at 460 K down to 350 K in a heat
exchanger (Figure E8-9.1).