Page 501 - Elements of Chemical Reaction Engineering Ebook
P. 501
472 Steady-State Nonisothermal Reactor Design Chap. 8
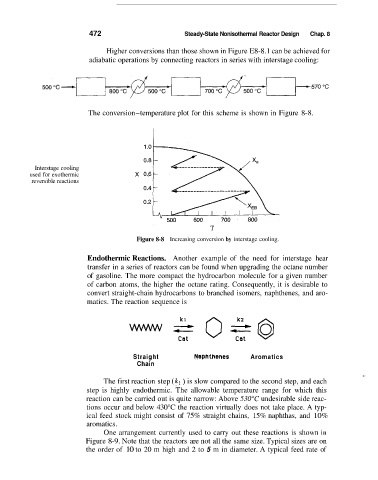
Higher conversions than those shown in Figure E8-8.1 can be achieved for
adiabatic operations by connecting reactors in series with interstage cooling:
The conversion-temperature plot for this scheme is shown in Figure 8-8.
1
Interstage cooling
used for exothermic
reversible reactions
T
Figure 8-8 Increasing conversion by interstage cooling.
Endothermic Reactions. Another example of the need for interstage hear
transfer in a series of reactors can be found when upgrading the octane number
of gasoline. The more compact the hydrocarbon molecule for a given number
of carbon atoms, the higher the octane rating. Consequently, it is desirable to
convert straight-chain hydrocarbons to branched isomers, naphthenes, and aro-
matics. The reaction sequence is
Straight Naphthenes Aromatics
Chain
The first reaction step (k, ) is slow compared to the second step, and each
step is highly endothermic. The allowable temperature range for which this
reaction can be carried out is quite narrow: Above 530°C undesirable side reac-
tions occur and below 430°C the reaction virtually does not take place. A typ-
ical feed stock might consist of 75% straight chains, 15% naphthas, and 10%
aromatics.
One arrangement currently used to carry out these reactions is shown in
Figure 8-9. Note that the reactors are not all the same size. Typical sizes are on
the order of 10 to 20 m high and 2 to 5 m in diameter. A typical feed rate of