Page 499 - Elements of Chemical Reaction Engineering Ebook
P. 499
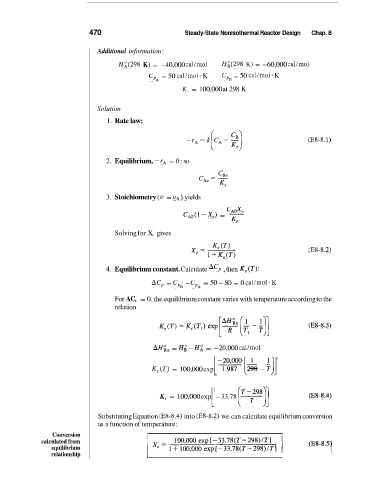
470 Steady-State Nonisothermal Reactor Design Chap. 8
Additional information:
Hi(298 K) = -40,000 cal/mol Hi(298 K) = -60,000 cal/mol
CpA = 50 cal/mol. K CpB = 50 cal/mol. K
K, = 100,000 at 298 K
Solution
1. Rate law:
(E8-8.1)
2. Equilibrium, -rA = 0; so
3. Stoichiometry (u = un ) yields
CAJe
C*n(l-xe) = -
K,
Solving for X, gives
(E8-8.2)
4. Equilibrium constant. Calculate AC, , then Ke(T):
ACp = CpB - CpA = 50 - SO = 0 cal/mol. K
For AC, = 0, the equilibrium constant varies with temperature according to the
relation
(E8-8.3)
AHix = Hi - Hi = -20,000 cal/mol ,-
;]I
K,(T) = 100,000 exp [ A - -
-:09:?~[2$.8
-
[ (T8)]
-
K, = 100,000 exp -33.78 (E8-8.4)
Substituting Equation (E8-8.4) into (E8-8.2) we can calculate equilibrium conversion
as a function of temperature:
Conversion I
calculated from
equilibrium
relationship