Page 533 - Elements of Chemical Reaction Engineering Ebook
P. 533
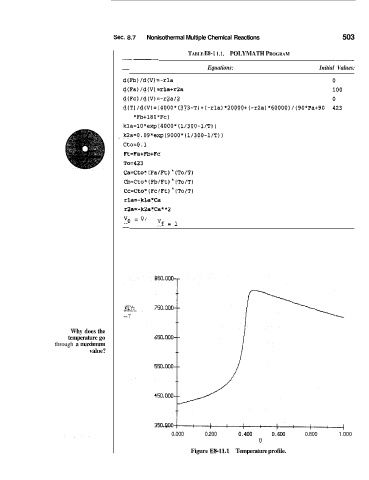
Sec. 8.7 Nonisothermal Multiple Chemical Reactions 503
-- - TABLE E8- 1 1.1. POLYMATH PROGRAM -
- Equations: Initial Values:
d(Fb) /d(V)=:-rla 0
d(Fa) /d(V) zrlatr2a 100
d(Fc) /d(V)=-r2a/2 0
d(T)/d(V)=(4000*(373-T)t(-r1a)*20000t(-r2a)*60000)/(90*Fa+90 423
*Fb+l80+Fc)
kla=lO*exp14000* (1/30O-l/T) )
~ kZa=0.09*exp(9000*(1/300-1/T))
Ct.o=O .1
Ft.=FatFb+Fc
To=423
*
Ca=Cto* (Fa/Ft) (To/")
*
Cb=Cto* (Fb,lFt) (To/T)
*
Cc:=Cto* (Fc,/Ft) (To/T)
rl.a=-kla*Ca
r;!a= -k2a*Ca* *2
vg = 0. 'Jf = 1
-
Why does the
temperature go
through a maximum
value?
~ ~ i ~ ~ ~ o o ~ - ~
0.000 0.200 0.400 0.600 0.800 1.000
U
Figure ES-11.1 Temperature profile.